
Volume 3, Issue 1, 2025
Editorial
Characteristics of Diseases in Developing Countries
Snur Othman
In the urgent realm of global health, it is imperative to acknowledge and promptly address the distinct characteristics of diseases that afflict developing countries. These nations face a unique set of challenges that contribute to the burden of illness and significantly impact the quality of life for their populations. By promptly exploring the key features of diseases in developing countries, we can better understand the complex interplay between socioeconomic factors and health outcomes [1].
One of the primary characteristics of diseases in developing countries is their disproportionate impact on vulnerable populations. Socio-economic disparities, limited access to healthcare, inadequate sanitation infrastructure, and poor nutrition contribute to the heightened susceptibility of marginalized communities to preventable infectious diseases such as hydatid cysts, malaria, tuberculosis, and diarrheal illnesses. The lack of resources and infrastructure further exacerbates the spread of these diseases, creating a cycle of sickness and poverty [2,3].
Furthermore, the prevalence of neglected tropical diseases (NTDs) is a defining feature of the disease landscape in developing countries. These illnesses, which include dengue fever, Chagas disease, and schistosomiasis, predominantly affect populations in low-resource settings and often go unnoticed on the global health agenda. The burden of NTDs is compounded by limited funding for research and treatment, leaving affected communities without adequate support and interventions [4].
In addition to infectious diseases, non-communicable diseases (NCDs) are on the rise in developing countries, posing a dual burden of illness alongside contagious diseases. Factors such as urbanization, lifestyle changes, and an aging population contribute to the increasing prevalence of conditions like cardiovascular diseases, diabetes, and cancer. The inadequate healthcare infrastructure in many developing countries further hinders NCDs' prevention, diagnosis, and management, leading to poorer health outcomes for those affected [5].
Addressing the characteristics of diseases in developing countries requires a multi-faceted approach that encompasses strengthening the healthcare system, investing in public health infrastructure, and, most importantly, providing equitable access to essential medicines. By ensuring fair access to these medicines, we can work towards achieving health equity and reducing the burden of disease in developing countries.
Original Articles

Assessment of Chat-GPT, Gemini, and Perplexity in Principle of Research Publication: A Comparative Study
Ameer M. Salih, Jaafar Omer Ahmed, Dilan S. Hiwa, Abdulwahid M. Salih, Rawezh Q. Salih, Hemn A....
Abstract
Introduction
Many researchers utilize artificial intelligence (AI) to aid their research endeavors. This study seeks to assess and contrast the performance of three sophisticated AI systems, namely, ChatGPT, Gemini, and Perplexity when applied to an examination focused on knowledge regarding research publication.
Methods
Three AI systems (ChatGPT-3.5, Gemini, and perplexity) were evaluated using an examination of fifty multiple-choice questions covering various aspects of research, including research terminology, literature review, study design, research writing, and publication-related topics. The questions were written by a researcher with an h-index of 22, and it was later tested on two other researchers with h-indices of 9 and 10 in a double-blinded manner and revised extensively to ensure the quality of the questions before testing them on the three mentioned AI systems.
Results
In the examination, ChatGPT scored 38 (76%) correct answers, while Gemini and Perplexity each scored 36 (72%). Notably, all AI systems frequently chose correct options significantly: ChatGPT chose option (C) correctly 88.9% of the time, Gemini accurately selected option (D) 78.9% of the time, and Perplexity correctly picked option (C) 88.9% of the time. In contrast, other AI tools showed minor agreement, lacking statistical significance, while ChatGPT exhibited significant concordance (81-83%) with researchers' performance.
Conclusion
ChatGPT, Gemini, and Perplexity perform adequately overall in research-related questions, but depending on the AI in use, improvement is needed in certain research categories. The involvement of an expert in the research publication process remains a fundamental cornerstone to ensure the quality of the work.
Introduction
The work of John McCarthy is the foundation of modern artificial intelligence (AI) research. In 1956, at Dartmouth College, he introduced the phrase "artificial intelligence," marking the inception of formal AI research [1]. The emergence of AI was an innovative technological frontier, promising transformative impacts across diverse sectors. Recent years have witnessed significant strides in the AI domain, particularly in the refinement of chatbot technology. An increasingly prevalent notion suggests that AI, having surpassed human capabilities in several domains, holds promise for substantial advancements in the realm of research publications. AI stands poised to augment research writing, the accuracy of information retrieved, and referencing, thereby potentially revolutionizing the field [2].
Over the past few years, a multitude of AI tools have become readily accessible, providing a diverse array of services and functionalities. A notable instance of such an AI system is ChatGPT, an advanced language model crafted by OpenAI. It underwent training using a vast array of textual materials gathered from websites, literature, and diverse sources, engaging in language modeling tasks to enhance its capabilities. This attribute sets it apart as one of the most expansive and resilient language models ever devised, integrating an astonishing 175 billion parameters [3,4]. An additional AI system that has attracted attention is Gemini, previously identified as Google Bard, which is an AI-driven information retrieval apparatus with a sophisticated chatbot that utilizes a "native multimodal" approach to effectively process and adjust to various types of data like video, audio, and text [5,6]. Perplexity AI stands as an AI-powered research and conversational search engine, adept at responding to queries through the utilization of natural language predictive text. It synthesizes answers from web sources, accompanied by citations through embedded links within the text response [7]. Many researchers are known to utilize chatbots as aids in their research endeavors.
This study seeks to assess and contrast the performance of sophisticated AI systems—namely, ChatGPT, Gemini, and Perplexity—when applied to an examination focused on knowledge regarding research publication. It also aims to shed light on the current state of AI integration within the research publication process and identify opportunities for further development.
Methods
In this comparative investigation, we evaluated the performance of three distinct AI systems: ChatGPT-3.5, Gemini, and Perplexity. The assessment comprised 50 multiple-choice questions, each offering four options (A-D). The questions spanned various domains including eleven research terminology queries, six literature review inquiries, twelve study design probes, twelve research writing assessments, and nine publication-related investigations.
Initially, a researcher with an h-index of 22, identified as the second author in the manuscript, composed a set of sixty multiple-choice questions. Subsequently, two other researchers with h-indices of 14 and 16, mentioned as authors seven and ten respectively, underwent the examination in a double-blinded fashion. Following this phase, all three researchers collaborated to review and analyze both questions and answers. Ten questions were excluded due to their lack of clarity, leaving a total of fifty questions selected for the final examination version. These selected questions were unanimously agreed upon by the researchers as informative indicators of knowledge within the realm of research and its associated intricacies.
The questions were then uniformly inputted into each of the AI systems in March 2024, following a standardized protocol. This protocol involved initiating interactions with the AI systems by introducing a prompt starting with "Hello." Subsequently, each AI system received the same directive: "Please select the correct answer for the following multiple-choice questions." The questions were directly transcribed from a prepared Word document, and the AI-generated responses were recorded in an Excel spreadsheet. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 27.0, with a significance level set at p < 0.05. Chi-square (Fisher's Exact Test) was employed for data analysis.
During the literature review phase of the present study, papers were selectively included from reputable journals and omitted those published in predatory journals, adhering to the criteria delineated in Kscien’s list [8].
Results
In the examination, ChatGPT demonstrated slightly higher accuracy with a total of 38 correct answers (76%), compared to 36 correct answers (72%) by both Gemini and Perplexity. Notably, Researcher 2 excelled in terminology and literature review questions, with 15 correct answers (88.23%), surpassing ChatGPT and Gemini, with 13 correct answers (76.47%). In research writing, Perplexity, along with Researcher 1 and Researcher 2, led with 10 correct responses (83.3%). Additionally, Researcher 1 exhibited the highest accuracy in research publication, with 9 correct responses (100%), outperforming ChatGPT and Researcher 2, who achieved 7 correct responses (77.78%) (Supplementary 1).
In the examination comparing AI tools and two researchers' accuracy in identifying correct answers, researchers demonstrated superior accuracy compared to AI tools. For example, in questions where the correct answer was C, Researcher 2 achieved a perfect 100% accuracy, outperforming ChatGPT, Perplexity, and Gemini, which scored 88.9%, and 77.8% respectively. Notably, all AI systems significantly chosen the correct options. For instance, ChatGPT correctly identified option C 88.9% of the time, Gemini correctly chose option D 78.9% of the time, and Perplexity accurately selected option C 88.9% of the time (Table 1).
| Correct | ChatGPT | ||||
| A | B | C | D | Total | |
| A |
7 (63.6%) |
0 (0.0%) |
2 (18.2%) |
2 (18.2%) |
11 (100%) |
| B |
0 (0.0%) |
8 (72.7%) |
2 (18.2%) |
1 (9.1%) |
11 (100%) |
| C |
0 (0.0%) |
0 (0.0%) |
8 (88.9%) |
1 (11.1%) |
9 (100%) |
| D |
0 (0.0%) |
3 (15.8%) |
1 (5.3%) |
15 (78.9%) |
19 (100%) |
| Total |
7 (14%) |
11 (22%) |
13 (26%) |
19 (38%) |
50 (100%) |
| P-value | <0.001 | ||||
| Correct | Gemini | ||||
| A | B | C | D | Total | |
| A |
7(63.6%) |
2(18.2%) |
1(9.1%) |
1(9.1%) |
11(100%) |
| B |
1(9.1%) |
7(63.6%) |
2(18.2%) |
1(9.1%) |
11(100%) |
| C |
0(0.0%) |
0(0.0%) |
7(77.8%) |
2(22.2%) |
9(100%) |
| D |
2(10.5%) |
2(10.5%) |
0(0.0%) |
15(78.9%) |
19(100%) |
| Total |
10(20%) |
11(22%) |
10(20%) |
19(38%) |
50(100%) |
| P-value | <0.001 | ||||
| Correct | Perplexity | ||||
| A | B | C | D | Total | |
| A |
8(72.7%) |
0(0.0%) |
1(9.1%) |
2(18.2%) |
11(100%) |
| B |
2(18.2%) |
5(45.5%) |
2(18.2%) |
2(18.2%) |
11(100%) |
| C |
0 (0.0%) |
0 (0.0%) |
8 (88.9%) |
1 (11.1%) |
9 (100%) |
| D |
0 (0.0%) |
3 (15.8%) |
1 (5.3%) |
15 (78.9%) |
19 (100%) |
| Total |
10 (20%) |
8 (16%) |
12 (24%) |
20 (40%) |
50 (100%) |
| P-value | <0.001 | ||||
| Correct | Researcher 1 | ||||
| A | B | C | D | Total | |
| A |
10 (90.9%) |
0 (0.0%) |
0 (0.0%) |
1 (9.1%) |
11(100%) |
| B |
0 (0.0%) |
9 (81.8%) |
0 (0.0%) |
2 (18.2%) |
11(100%) |
| C |
0 (0.0%) |
1 (11.1%) |
8 (88.9%) |
0 (0.0%) |
9(100%) |
| D |
0(0.0%) |
2 (10.5%) |
1 (5.3%) |
16 (84.2%) |
19 (100%) |
| Total |
10 (20%) |
12 (24%) |
9 (18%) |
19 (38%) |
50 (100%) |
| P-value | <0.001 | ||||
| Correct | Researcher 2 | ||||
| A | B | C | D | Total | |
| A |
10 (90.9%) |
0 (0.0%) |
0 (0.0%) |
1 (9.1%) |
11 (100%) |
| B |
1 (9.1%) |
9 (81.8%) |
1 (9.1%) |
0 (0.0%) |
11 (100%) |
| C |
0 (0.0%) |
0 (0.0%) |
9 (100%) |
0 (0.0%) |
9 (100%) |
| D |
2 (10.5%) |
1 (5.3%) |
3 (15.8%) |
13 (68.4%) |
19 (100%) |
| Total |
13 (26%) |
10 (20%) |
13 (26%) |
14 (28%) |
50(100%) |
| P-value | <0.001 | ||||
In comparing AI tools and researchers' performance, significant agreement was noted with ChatGPT. For instance, out of 43 questions where researcher 1 agreed on the correct answer, ChatGPT agreed in 35 cases (81.4%) and disagreed in only 8 answers (18.6%). However, the comparison with the other two AI tools showed no significance but a slight alignment with the researchers' agreement on the correct answers (Table 2).
| AI tools | Researcher 1 | P-value | Researcher 2 | P-value | ||
| Agree | Disagree | Agree | Disagree | |||
|
ChatGPT 3.5 |
0.048 | 0.027 | ||||
|
Agree |
35 (81.4%) |
3 (42.9%) |
34 (82.9%) |
4 (44.4%) |
||
|
Disagree |
8 (18.6%) |
4 (57.1%) |
7 (17.1%) |
5 (55.6%) |
||
|
Total |
43 (100%) |
7 (100%) |
41 (100%) |
9 (100%) |
||
|
Gemini |
0.300 | 0.697 | ||||
|
Agree |
32 (74.4%) |
4 (57.1%) |
30 (73.2%) |
6 (72%) |
||
|
Disagree |
11 (25.6%) |
3 (42.9%) |
11 (26.8%) |
3 (33.3%) |
||
|
Total |
43 (100%) |
7(100%) |
41 (100%) |
9 (100%) |
||
|
Perplexity |
0.085 | 0.094 | ||||
|
Agree |
33 (76.7%) |
3 (42.9%) |
32 (78%) |
4 (44.4%) |
||
|
Disagree |
10 (23.3%) |
4 (57.1%) |
9 (22%) |
5 (55.6%) |
||
|
Total |
43 (100%) |
7 (100%) |
41 (100%) |
9 (100%) |
||
|
*Fisher's Exact Test |
||||||
Discussion
The imitation of human intelligence functions by machines, most commonly computer systems, is referred to as AI. It involves acquiring knowledge (gaining information and understanding rules for its utilization), logical deduction (applying rules to arrive at rough or precise outcomes), and self-adjustment. In addition, AI endeavors to develop systems capable of executing tasks traditionally associated with human intelligence, including decision-making, speech recognition, language translation, and visual perception, among various others [9]. Although AI language models have been in development for years, the general population's understanding of AI's potential and use has increased dramatically recently. The academic community has already embraced language-based AI, and numerous researchers utilize chatbots as aids in their research. These bots assist in structuring ideas, offering feedback on their work, and aiding in referencing and summarizing the existing research literature [2,10,11].
Kacena et al. demonstrated that the utilization of AI, particularly ChatGPT, reduced the time invested in crafting review articles. However, it yielded the highest similarity indices, indicating a greater probability of plagiarism. In addition, they reported that ChatGPT possesses the ability to swiftly scour the internet and evaluate potential sources, potentially accelerating the literature review process. In the current study, the performance of ChatGPT regarding the principle of literature review questions showed a high performance, and Gemini scored just as high, further supporting the finding of the previous study [12].
Salvagno et al. reported that AI may soon be leveraged for the automated production of figures, tables, and supplementary visual components within manuscripts. This utilization could facilitate data summarization and contribute to manuscript lucidity [13]. However, the current study demonstrated that the AI systems had different scores, and their performance was influenced by the different categories they were tested on, which means that identifying the strengths and weaknesses of the currently available AIs is paramount in choosing which AI system will aid in research publications rather than hindering and jeopardizing the integrity of the research paper, For instance, Kacena et al. showcased that 70% of the references were incorrect when an AI only method was applied to writing research papers, raising controversy if these AI tools should even be used as aid in that regard [12]. The present study showed that Gemini performed poorly by only getting half of the questions wrong in the research writing principles questions. In addition, Perplexity was shown to perform poorly on principles of publication-related questions, and ChatGPT exhibited subpar performance in research terminology inquiries, further supporting the notion that leveraging AI use is dependent on recognizing their limitations in the field of research.
Concerns about biases in AI systems, stemming from their training data, are widely recognized as a significant challenge. Research indicates that AI models can perpetuate biases and exhibit skewed behavior, replicating existing discriminatory patterns. Addressing these biases is crucial and requires the implementation of effective strategies prioritizing fairness and justice during development. This is particularly important in research, where ensuring impartiality is paramount. Responsible use of advanced language models like ChatGPT, Gemini, and Perplexity is essential, given the ethical dilemmas they pose, including the potential for misinformation and emotionally persuasive content. Proactive steps are needed to mitigate these risks and promote responsible usage. Additionally, the use of AI in content generation raises concerns about unintentional plagiarism, as systems may reproduce text without proper citation. While AI tools may increase publication output, there may not be a corresponding increase in expertise or experience among researchers [3,12].
Several studies have investigated the comparison of AI and human capabilities across various domains. Long et al. noted a remarkable level of accuracy in AI, ranging from 90% to 100% when evaluating its performance against specialized doctors' diagnostic and treatment decisions for congenital cataracts [14]. Additionally, Rajpurkar et al. discovered consistency in results between AI and radiologists, particularly in diagnosing chest radiographs [15]. However, there is limited available data on the comparison of AI and human performance in research principles. In this study, the comparison between AI tools and human performance regarding predetermined correct answers on research principles revealed a significant agreement (80-85%) between ChatGPT and researchers.
One of the limitations of our study is that we evaluated only three AI systems in comparison to the vast and increasing number of AI tools becoming available in these times. In addition, a larger number of questions will lead to a more comprehensive understanding of the strengths and weaknesses of these AI systems in the field of research and their utilities in that regard.
Conclusion
ChatGPT, Gemini, and Perplexity perform adequately overall in research-related questions, but depending on the AI in use, improvement is needed in certain research categories. The involvement of an expert in the research publication process remains a fundamental cornerstone to ensure the quality of the work.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: RQS and SHM were major contributors to the conception of the study and the literature search for related studies. AMS, JOA, DSH, and AMS were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. HAH, and YMM were involved in the literature review, study design, and manuscript writing. BAA, DSH, and RQS Literature review, final approval of the manuscript, and processing of the tables. RQS and SHM confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
Acknowledgement: Not applicable.

Clinicopathological Features of Indeterminate Thyroid Nodules: A Single-center Cross-sectional Study
Rebaz M. Ali, Abdulwahid M. Salih, Hiwa O. Abdullah, Ari M. Abdullah, Rawa M. Ali, Aras J....
Abstract
Introduction
Due to indeterminate cytology, Bethesda III is the most controversial category within the Bethesda System for Reporting Thyroid Cytopathology. This study examines the characteristics and malignancy rates of thyroid nodules (TNs) classified as Bethesda III.
Methods
Data were collected by reviewing electronic medical records, encompassing demographic details, medical history, chief complaint, laboratory tests (including thyroid function tests), preoperative imaging, cytology results, management, and histopathology diagnosis.
Results
The majority of the cases were female (84.7%). Patients’ ages ranged from 15 to 71 years, with a mean of 42.9 ± 10.5 years. Regarding goiter grading, 37 cases (21.8%) were classified as G0, 62 (36.5%) as G1, 55 (32.3%) as G2, and seven (4.1%) as G3. Thyroid Imaging Reporting and Data Systems scoring categorized the nodules as TI-RADS 2 (5.3%), TI-RADS 3 (40%), TI-RADS 4 (38.2%) and TI-RADS 5 (9.4%). The size of TNs on ultrasound ranged from 0.3 cm to 7.8 cm, with a mean size of 2.06 ± 1.3 cm. Adenoma was the most common diagnosis (40%), followed by thyroiditis (16.5%), papillary thyroid carcinoma (15.9%), and papillary thyroid microcarcinoma (15.9%). The nodules were predominantly benign (64.7%), while 35.3% were malignant. Patients with malignant nodules were younger than those with benign nodules (p=0.044). Benign nodules were significantly larger than malignant ones (p-value = 0.003).
Conclusion
One of three TNs with indeterminate cytology may be malignant. Patients with malignant nodules tend to be younger than those with benign nodules, and benign nodules are likely larger than malignant ones.
Introduction
The global incidence of thyroid nodules (TNs) is estimated to range between 20% and 60%, varying by gender, age, and geographic location [1]. Almost 90–95% of these nodules are benign and asymptomatic at diagnosis and remain so during follow-up [2]. However, the incidence of thyroid cancer, including papillary thyroid carcinoma (PTC) and papillary thyroid microcarcinoma (PTMC), has risen concurrently with the advancements in diagnostic technology and enhanced surveillance. Additionally, incidence-based mortality from thyroid cancer has increased, with an annual percent change of 1% [1,3]. With new ultrasound (U/S) technology and the widespread use of high-resolution scanners, detecting TNs has become much easier. However, for many sonographers, the primary challenge lies in accurately distinguishing malignant TNs from benign ones. To address this, certain U/S characteristics, such as unclear borders, micro-calcifications, irregular shapes, solid components, and internal echoes, are commonly used to assess the malignancy risk of nodules. Nonetheless, relying on any single characteristic alone is insufficient to accurately differentiate between malignant and benign nodules [4]. Fine needle aspiration cytology (FNAC) has become the standard modality for assessing thyroid nodular pathology [1]. In 2008, the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) was introduced to standardize the cytological evaluation of TNs. The BSRTC categorizes diagnoses into six classes with progressively higher suspicion for malignancy: nondiagnostic (Class I), benign (Class II), atypia of undetermined significance /follicular lesion of undetermined significance (AUS/FLUS) (Class III), follicular neoplasm/oncocytic cell neoplasm (Class IV), suspicious for malignancy (Class V), and malignant (Class VI) [5]. The most controversial category within the BSRTC is AUS/FLUS due to indeterminate cytology. Despite the routine use of cytological examination in evaluating TNs, which has reduced the overall number of patients needing diagnostic surgery, a significant percentage still undergo surgery to obtain a definitive histological diagnosis [1]. The reported malignancy rates for AUS and FLUS exhibit considerable variability [3]. This study examines the characteristics and malignancy rates of TNs classified as Bethesda III. The referenced studies have been checked to avoid citing non-peer-reviewed data [6].
Methods
Study design
This retrospective, single-center, cross-sectional study was conducted at Smart Health Tower between August 2024 and September 2024. The patients gave verbal informed consent to publish their data in this study. The ethical board at Kscien organization approved the study with approval number 26 on August 2024.
Data collection
Data were collected by reviewing electronic medical records, encompassing demographic details, medical history, chief complaints, laboratory tests (including thyroid function tests), preoperative imaging, FNAC results, management, and final histological diagnosis.
Eligibility criteria
The study included patients with TNs classified as Bethesda III on FNAC who subsequently underwent surgery for a definitive histopathological diagnosis. Patients with incomplete medical documentation, including clinical, radiological, and FNAC data, as well as those with recurrent or a history of thyroid cancer, were excluded.
Statistical Analysis
The data were collated in a Microsoft Excel (2021) sheet and then transferred into version 27 of Statistical Package for Social Sciences (SPSS). The chi-squared and Fisher's exact tests were used to analyze categorical data with independent samples t-test for quantitative variables. The data were presented as frequency, percentage, mean with standard deviation, and median with ranges. The level of significance was set at p-value <0.05.
Results
Patient demography and presentation
The study included 170 patients, of whom the majority were female (84.7%). Their ages ranged from 15 to 71 years, with a mean of 42.9 ± 10.5. In total, 157 patients (92.3%) were married, 11 (6.5%) were unmarried, and two individuals (1.2%) were either divorced or widowed. Most of the cases (65.9%) were housewives. Seven cases were smokers (4.1%), while 20 were passive smokers (11.8%), and one (0.6%) was an ex-smoker. The reason for the presentation was having a thyroid disease and visiting the hospital for follow-up in most of the cases (38.2%), followed by neck swelling (34.7%) and fatigue (10.0%) (Table 1). Regarding goiter grading, 37 cases (21.8%) were classified as G0, 62 (36.5%) as G1, 55 (32.3%) as G2, and seven (4.1%) as G3. Nine patients (5.3%) had no goiter grading available (Table 2).
|
Variables |
Frequency / Percentage |
|
Patient demographics |
|
|
Age range (median, mean ± SD), years |
15 – 71 (43, 42.9 ± 10.5) |
|
Sex Male Female |
26 (15.3%) 144 (84.7%) |
|
Marital status Unmarried Married Divorced/Widow |
11 (6.5%) 157 (92.3 %) 2 (1.2%) |
|
Occupation Housewife Teacher Worker Unemployed Policeman Student Doctor Retired Others |
112 (65.9%) 16 (9.4%) 15 (8.8%) 7 (4.1%) 4 (2.3 %) 3 (1.8%) 2 (1.2%) 2 (1.2%) 9 (5.3%) |
|
Smoking status Smoker Passive smoker Ex-smoker Non-smoker |
7 (4.1%) 20 (11.8%) 1 (0.6%) 142 (83.5%) |
|
Chief complaint Follow-up Neck swelling Fatigue Neck pain Palpitation Dysphagia Exophthalmos Hair loss Sweating Weigh gain N/A |
65 (38.2%) 59 (34.7%) 17 (10.0%) 10 (5.9 %) 4 (2.3 %) 3 (1.8 %) 3 (1.8 %) 2 (1.2 %) 1 (0.6 %) 1 (0.6 %) 5 (2.9%) |
|
Variables |
Frequency / Percentage |
|
Goiter grading G0 G1 G2 G3 N/A |
37 (21.8 %) 62 (36.5 %) 55 (32.3 %) 7 (4.1%) 9 (5.3 %) |
|
Thyroid state Euthyroid Hypothyroidism Hyperthyroidism N/A |
87 (51.2 %) 42 (24.7%) 29 (17.1%) 12 (7.0%) |
|
Feature on ultrasound Solid Cystic Mixed N/A |
113 (66.5 %) 1 (0.6 %) 32 (18.8%) 24 (14.1%) |
|
TI-RADS score TR2 TR3 TR4 TR5 N/A |
9 (5.3%) 68 (40.0%) 65 (38.2%) 16 (9.4%) 12 (7.1%) |
|
Tumor size on ultrasound (range, mean ± SD), cm |
0.3 - 7.8, 2.06 ± 1.3 |
|
Tumor size group <1 cm 1-2 cm >2-3 cm >3-4 cm >4 cm N/A |
35 (20.6%) 61 (35.9%) 34 (20.0%) 22 (12.9 %) 15 (8.8%) 3 (1.8 %) |
|
Fine needle aspiration Bethesda III |
170 (100.0%) |
|
Management Total thyroidectomy Lobectomy Nodulectomy Isthmusectomy |
115 (67.6 %) 30 (17.6 %) 21 (12.4 %) 4 (2.4 %) |
|
Diagnosis Adenoma Thyroiditis PTC PTMC MNG NIFTP FTC Graves’ disease Collision tumor (PTMC and follicular adenoma) MTC |
68 (40.0%) 28 (16.5 %) 27 (15.9 %) 27 (15.9 %) 7 (4.1%) 5 (2.9%) 4 (2.3%) 2 (1.2 %) 1 (0.6 %) 1 (0.6 %) |
|
Nature of tumor Benign Malignant |
110 (64.7 %) 60 (35.3 %) |
|
N/A: Non-available, PTMC: Papillary thyroid microcarcinoma, PTC: Papillary thyroid carcinoma, FTC: Follicular thyroid carcinoma, MTC: Medullary thyroid carcinoma, MNG: multinodular goiter, NIFTP: Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. |
|
Diagnosis and management
Thyroid function assessment revealed that the majority of the participants were euthyroid (51.2%). However, hypothyroidism and hyperthyroidism were observed in 24.7% and 17.1% of patients, respectively. Ultrasonography of the thyroid showed that 113 patients (66.5%) had solid nodules, while 32 (18.8%) presented with mixed (solid and cystic) nodules. Only one case (0.6%) had a purely cystic nodule. The content of the tumor in the remaining cases was unknown (14.1%). Thyroid Imaging Reporting and Data Systems (TI-RADS) scoring categorized the nodules as TI-RADS 2 (5.3%), TI-RADS 3 (40%), TI-RADS 4 (38.2%) and TI-RADS 5 (9.4%). It was unknown in 12 cases (7.1%). The size of the TNs on U/S ranged from 0.3 cm to 7.8 cm, with a mean of 2.06 ± 1.3 cm. The majority of the cases were managed by total thyroidectomy (67.6%), followed by lobectomy (17.6%), nodulectomy (12.4%), and isthmusectomy (2.4%). Adenoma (Figure 1) was the most common diagnosis, identified in 68 cases (40%), followed by thyroiditis (16.5%), PTC (15.9%) (Figure 2), and PTMC (15.9%) (Figure 3). Benign multinodular goiter (MNG) was diagnosed in seven individuals (4.1%), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in five patients (2.9%) (Figure 4), and follicular thyroid carcinoma (Figure 5) in four cases (2.3%). Other less common diagnoses included Graves’ disease in two patients (1.2%), a collision tumor consisting of PTMC and follicular adenoma in one individual (0.6%), and medullary thyroid carcinoma in another patient (0.6%). The nodules were predominantly benign (64.7%), while 35.3% were malignant (Table 2).
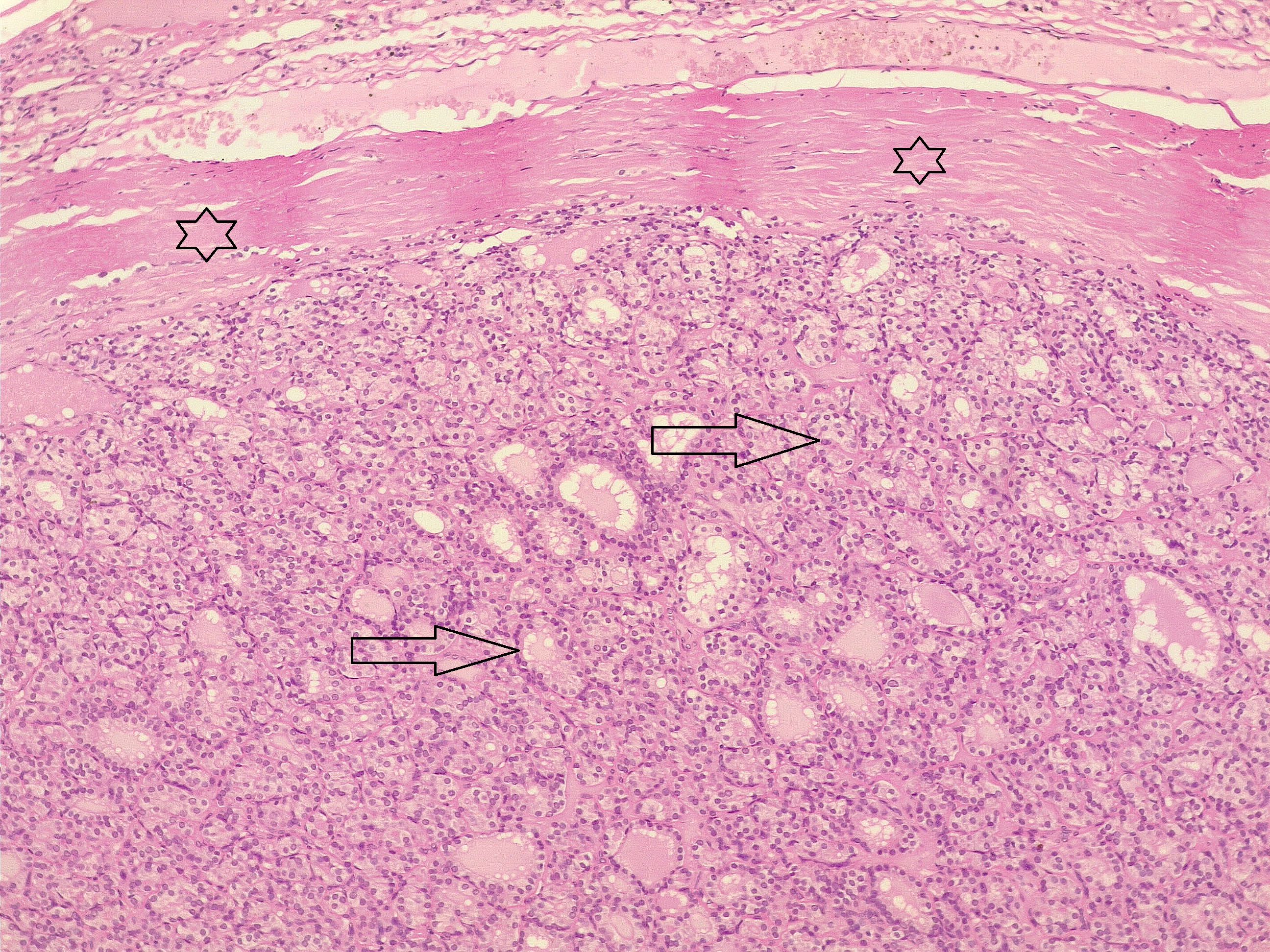
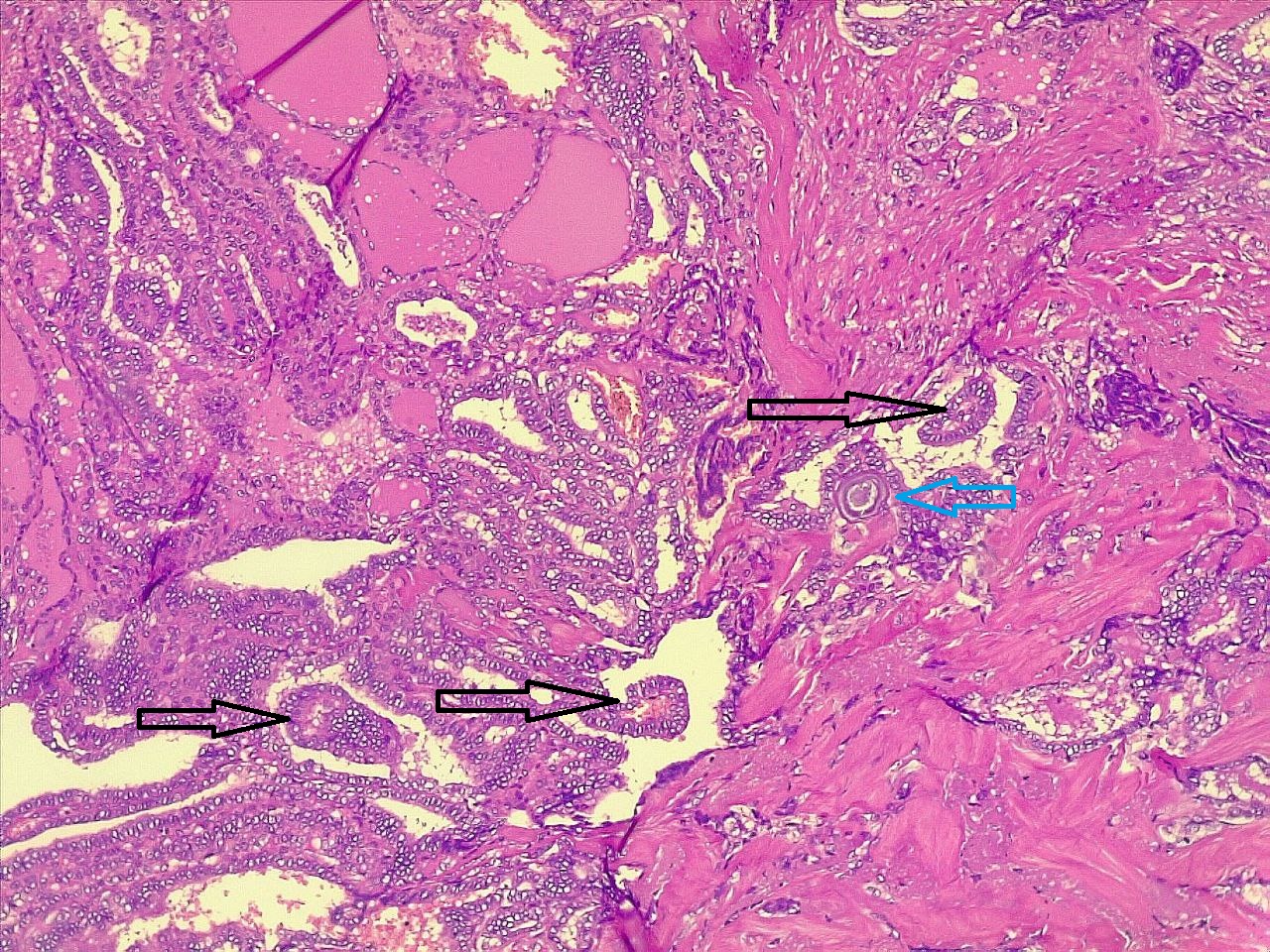
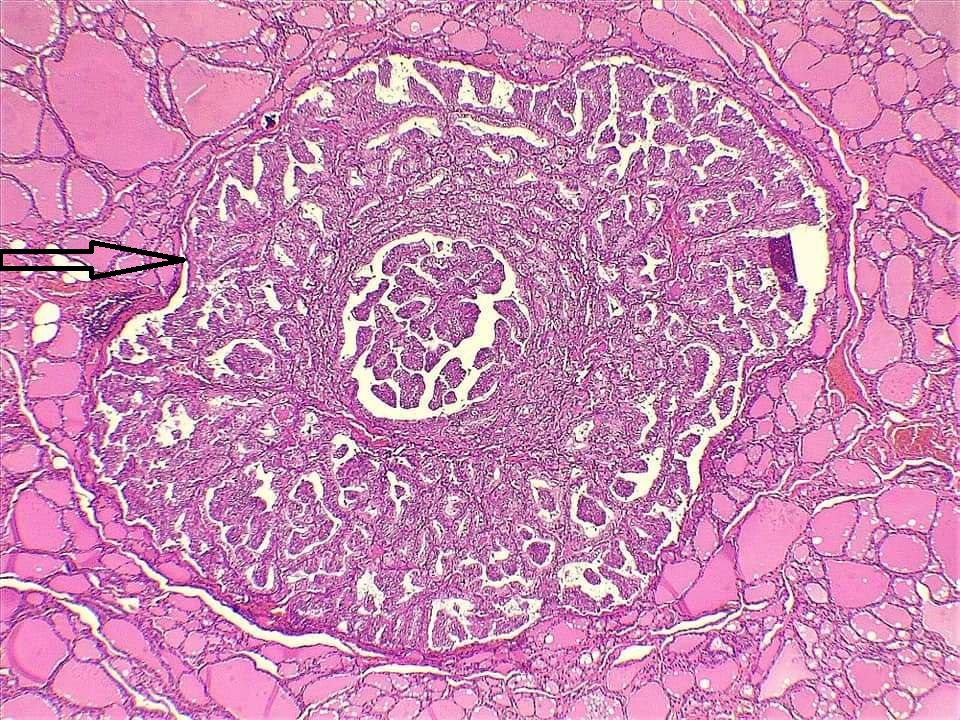
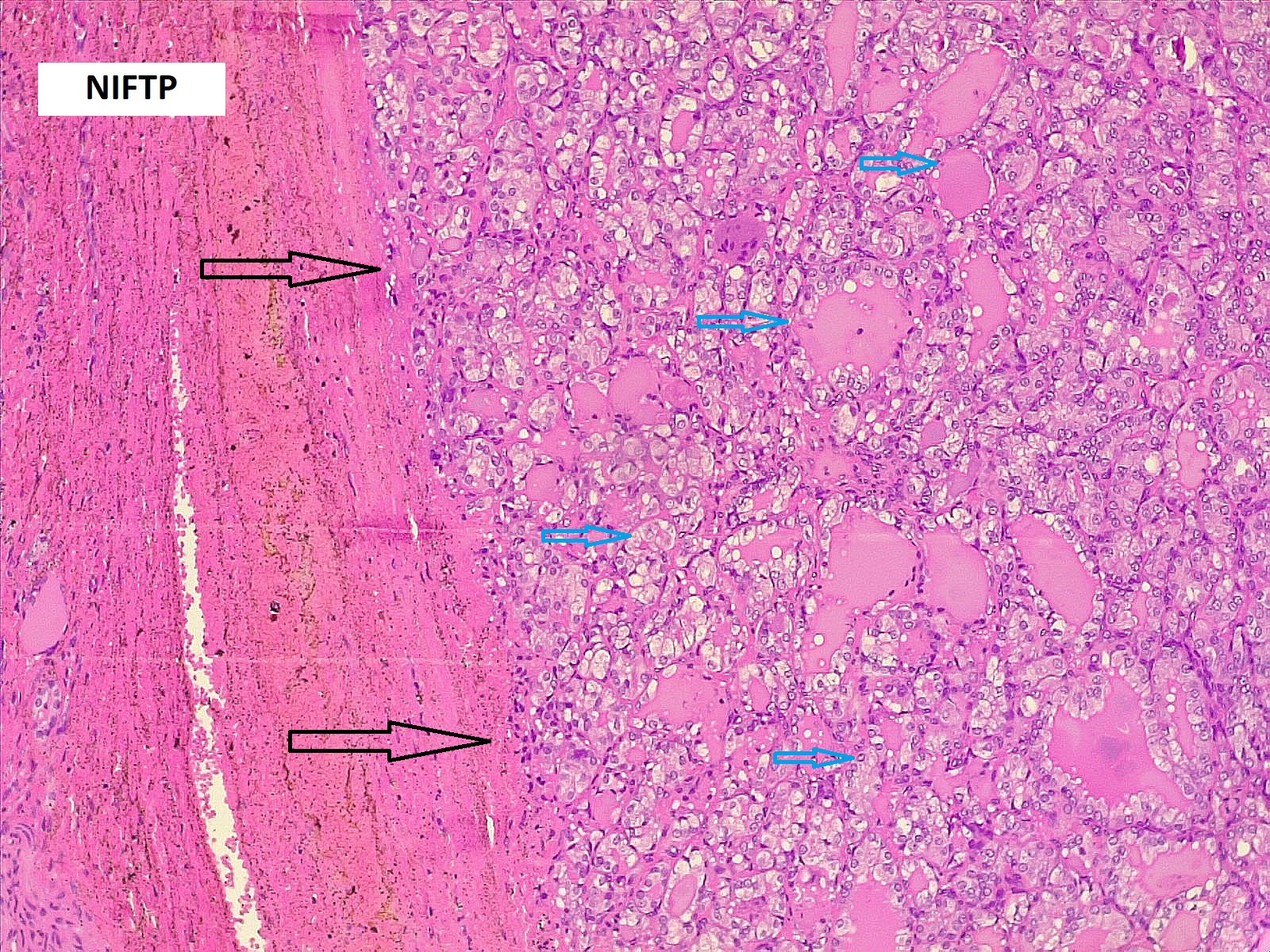
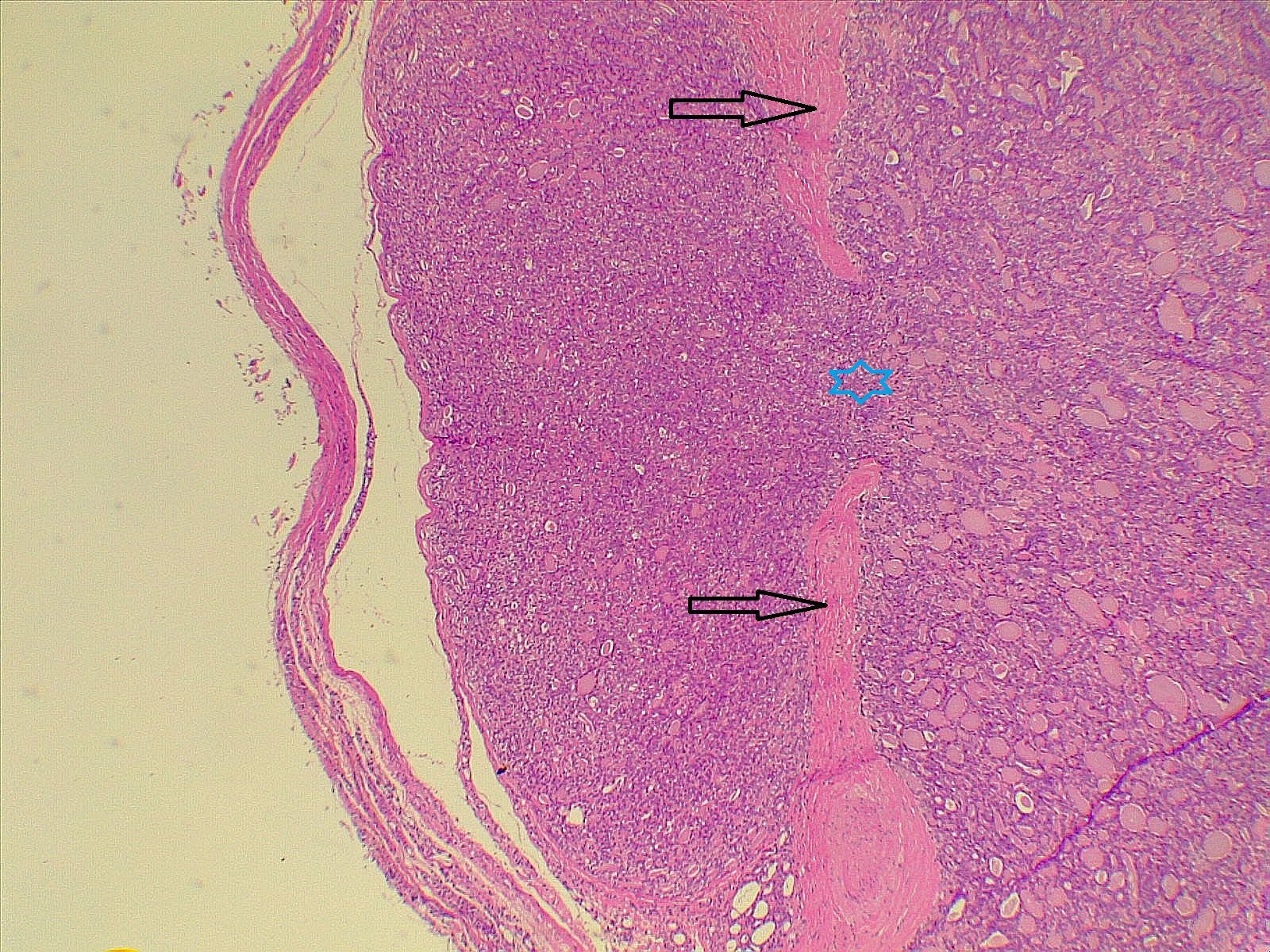
Comparison of patient and tumor characteristics between benign and malignant TNs
In comparing patients' demography and clinical, and radiological characteristics between benign and malignant nodules, no significant differences were observed between the two groups concerning age group, gender, goiter grading, MNG (data not shown), thyroid function, or tumor features on U/S (p-value > 0.05). However, in general, patients with malignant nodules tended to be younger than those with benign nodules (p=0.044). TI-RADS classification differed significantly between benign and malignant nodules. Malignant nodules were significantly associated with TI-RADS categories 4 and 5, whereas benign nodules were predominantly associated with TI-RADS categories 2 and 3 (p-value < 0.001). There was no statistically significant difference in tumor size between benign and malignant nodules when categorized into groups (p-value = 0.053) (Table 3). However, when tumor size was not grouped, benign nodules were significantly larger than malignant ones (p-value = 0.003) (Table 4).
| Variables | Nature of tumor |
Total |
P-value* |
|
|
Benign |
Malignant |
|||
|
Age group 15-25 26-35 36-45 46-55 56-65 >65 |
5 14 42 35 12 2 |
5 12 27 11 4 1 |
10 26 69 46 16 3 |
0.29 |
|
Sex Male Female |
15 95 |
11 49 |
26 144 |
0.50# |
|
Goiter grading G0 G1 G2 G3 |
24 39 37 6 |
13 23 18 1 |
37 62 55 7 |
0.67 |
|
Thyroid state Euthyroid Hyperthyroidism Hypothyroidism |
51 21 31 |
36 8 11 |
87 29 42 |
0.15 |
|
Tumor feature on ultrasound Solid Cystic Mixed |
72 1 24 |
41 0 8 |
113 1 32 |
0.38 |
|
TI-RADS score TR2 TR3 TR4 TR5 |
3 50 43 2 |
1 16 23 14 |
4 66 66 16 |
<0.001 |
|
Tumor size group <1 cm 1-2 cm >2-3 cm >3-4 cm >4 |
17 40 23 15 14 |
18 21 11 7 1 |
35 61 34 22 15 |
0.053 |
|
*Chi-squared test, # Fisher's exact test |
||||
|
Variables |
Number |
Mean |
Std. Deviation |
Std. Error Mean |
P-value** |
|
Tumor size Benign Malignant |
110 58 |
2.28 1.64 |
1.41 1.01 |
0.14 0.13 |
0.003 |
|
Age Benign Malignant |
110 60 |
44.16 40.76 |
10.54 10.22 |
1.0 1.32 |
0.044 |
|
**Independent sample t-test |
|||||
Discussion
Although FNAC is widely utilized, its diagnostic accuracy ranges from 80% to 99%. The ambiguity associated with indeterminate cytological outcomes often leads to uncertainty, and repeating FNAC following a non-diagnostic result remains controversial [7]. Thyroid pathology, especially TNs, primarily affects women. The majority of thyroid cancer patients are women, with an average age of 50 ± 15 years. However, men are more likely to develop aggressive forms of the disease, which are linked to a poorer prognosis [8]. The global incidence of thyroid cancer is on the rise. In 2020, the estimated incidence rate was 10.1 per 100,000 people for women and 3.1 for men, up from 6.1 and 1.9, respectively, in 2012 [8]. Regarding the correlation of gender and age with the risk of malignancy in TNs with indeterminate cytology, controversial findings have been reported [1,3,8,9]. Bessey et al. reported female gender as a risk factor for malignancy [9]; however, Cozzani et al. found that female gender was associated with well-differentiated thyroid cancer predominantly in younger individuals, but this difference diminished in patients over the age of 55 [1]. In contrast, Rano et al. found no effect of gender, race, ethnicity, and underlying thyroid disease on thyroid cancer [8]. Several studies have identified a minimal inverse correlation between patient age and malignancy, suggesting that younger patients with indeterminate nodules face a slightly higher risk of being diagnosed with thyroid cancer [1,8-10]. On the contrary, Dimitriadis et al. reported a similar mean age of 50 between both groups of patients with benign and malignant nodules [3]. In line with existing literature, most of the cases in the present study were female (84.7%); however, gender did not differ significantly between those with malignant and benign nodules. Additionally, no significant difference between malignant and benign nodules was observed when age was categorized into groups. Nonetheless, patients with malignant nodules tended to be younger, with a mean age of 40.76 years, compared to those with benign nodules, who had a mean age of 44.16 years. This finding supports the assumption that TNs in younger patients are more likely to be malignant.
Some scholars have identified iodine deficiency as an indirect risk factor for thyroid cancer. Iodine plays a crucial role in the synthesis of thyroid hormones, and its deficiency can lead to an increase in thyroid volume (goiter) and elevated thyroid-stimulating hormone production. Rano et al. reported a high incidence of goiter among their cases (63%), with malignant histology more commonly associated with MNG (71%) than a single nodule. They found no difference between benign and malignant tumors regarding nodule content (solid, cystic, mixed) on U/S [8]. In the present study, only seven cases (4.1%) had MNG, and contrary to the previous study, there was no association between MNG and an increased risk of malignancy. However, consistent with the findings of Rano et al. [8], nodule content did not differ based on tumor nature, whether benign or malignant.
The usefulness of nodule size as an independent predictor of malignancy remains controversial. Both the British Thyroid Association and the American Thyroid Association recommend total thyroidectomy for indeterminate lesions measuring ≥40 mm due to the associated increased risk of malignancy [11,12]. In a study by Dimitriadis et al., the average nodule size was comparable between benign and malignant subgroups, measuring under 4 cm (3.5 cm vs. 3 cm, respectively). The study also found that the likelihood of a nodule being malignant was similar regardless of its size, whether it was <4 cm or ≥4 cm (27% and 27.7%, respectively) [3]. In line with these findings, some other studies also identified no correlation between nodule size and the risk of malignancy [1,13,14]. Despite that, some others reported different findings. A study from Oxford found that approximately 37% of TNs classified as Bethesda III and measuring over 4 cm were malignant, which was significantly higher than the malignancy rate observed in nodules smaller than 4 cm [15]. Conversely, Cavallo et al. found that larger nodules had a lower malignancy rate and suggested that nodule size should not be considered an independent risk factor for malignancy [16].
Cozzani et al. reported that only 11.6% of the nodules in their study were larger than 4 cm. They attributed this finding to the extensive use of U/S examinations, including those conducted for screening purposes, in their area, which has a very high incidence of thyroid nodular pathology [1]. In the current study, only 8.8% of the cases had nodules greater than 4 cm, which may be due to the reason mentioned by Cozzani et al [1]. Our findings were consistent with the presumption that tumor size in benign nodules may be greater than that of malignant ones.
The literature demonstrates considerable variability in the reported malignancy rates for AUS/FLUS TNs. In a study by Dimitriadis et al., the rate was about 34%, and in the 2017 National British Association of Endocrine and Thyroid Surgeons Audit report, it was 25.7% [3,17]. The Oxford group found that approximately one in four patients with AUS/FLUS cytology was diagnosed with thyroid cancer [15]. Similar results were observed in a systematic review that included 13 studies, revealing a malignancy rate of 22% for cases with Bethesda III cytology [18]. Additionally, malignancy rates of 34.9% and 39% have also been reported [8,19]. In the current study, the malignancy rate among Bethesda III nodules was 35.3%, comparable to what has been reported in the literature.
In a study by Bresler et al., 9% of Bethesda III nodules were histologically malignant, with 50% of these being PTC and 30% PTMC [20]. Another study found PTC as the predominant histological type among malignant TNs [8], while Finlayson et al. reported follicular carcinoma as the predominant type [21]. In this study, PTC and PTMC were the most common cancer types among the malignant nodules, with equal incidence (15.9%).
The primary objective of thyroid surgery for nodules classified as AUS/FLUS is to achieve a definitive histological diagnosis while ensuring complete removal of the pathological nodule. This approach aims to facilitate optimal surgical and medical management, thereby minimizing the risks associated with excessive surgical intervention and related adverse events [1]. Surgical options for TNs, such as total thyroidectomy, lobectomy, or nodulectomy, are influenced by several factors. These include risk factors indicating a higher likelihood of malignancy (such as nodules larger than 4 cm, a family history of neoplasia, and a history of radiation exposure), U/S characteristics, cytological category, and molecular testing. Additionally, these risk factors should be considered in conjunction with the patient’s preferences, the presence of contralateral nodularity, possible coexisting hyperthyroidism, and any comorbid conditions [22]. It is important to note that total thyroidectomy is no longer universally recommended for all differentiated carcinomas larger than 1 cm. According to the 2015 ATA Guidelines, lobectomy may be an adequate initial treatment for differentiated carcinomas smaller than 4 cm, NIFTP of any size, minimally invasive follicular carcinomas, and encapsulated or intrathyroidal variants of papillary carcinomas [1,22]. Another study recommended that nodulectomy may be an appropriate option for managing large, solitary TNs and small suspicious nodules or microcarcinomas [23]. In the present study, 67.6% of the cases underwent total thyroidectomy, with lobectomy in 17.6%, nodulectomy in 12.4%, and isthmusectomy in 2.4%. The present study's limitations included a small sample size and the retrospective nature of data collection, which may have led to the omission of important data, such as details on ultrasonography.
Conclusion
One of three TNs with indeterminate cytology may be malignant. Patients with malignant nodules tend to be younger than those with benign nodules, and benign nodules could be larger. While total thyroidectomy is common, lobectomy and nodulectomy may be viable alternatives for specific cases, emphasizing the need for individualized treatment.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The study's ethical approval was obtained from the scientific committee of the Kscien Organization for Scientific Research.
Patient consent (participation and publication): Verbal informed consent was obtained from patients for publication.
Source of Funding: Smart Health Tower.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: AMS and AMA were significant contributors to the conception of the study and the literature search for related studies. RMA, AJQ, ROM and RJR were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. HOA, HOB, and AAQ were involved in the literature review, study design, and manuscript writing. HAA and SHH Literature review, final approval of the manuscript, and processing of the tables. RMA was the pathologist who performed the histopathological diagnosis. HOA and RMA confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.

Evolution of Antimicrobial Resistance in Community vs. Hospital-Acquired Infections
Ayman M. Mustafa, Rawezh Q. Salih, Hidayat A. Yaseen, Wafa A. Hamadameen, Suhaib H. Kakamad,...
Abstract
Introduction
Hospitals are high-risk environments for infections. Despite the global recognition of these pathogens, few studies compare microorganisms from community-acquired and hospital-acquired infections (HAIs). This study compares these microorganisms and explores their relationship with patients' comorbidities and socio-demographic factors.
Methods
This retrospective cross-sectional study was conducted at Smart Health Tower, Iraq, from January to December 2023, focusing on patients with community-acquired infections and HAIs. Data were extracted from microbiology laboratory records, including blood cultures, urine samples, and other body fluids, with patients classified based on CDC and IDSA guidelines. Bacterial identification combined conventional methods and the BD Phoenix™ M50 system, while antibiotic susceptibility was tested using the Kirby-Bauer method and the same automated system. Statistical analysis of resistance patterns utilized SPSS version 25, with significance set at p ≤ 0.05.
Results
In this study of 2,157 participants, 1,303 (60.4%) were male, with microbial growth observed in 1,177 cases (54.6%). Notably, 41.1% of females and 52.1% of males showed no growth (p < 0.001). The mean age was 43.62 ± 23.3 years. Wound samples had the highest growth rate (91.2%), while body fluids had the lowest (33.0%) (p < 0.001). The overall multidrug resistance rates were 62.5% for community-acquired infections and 79.3% for HAIs. Patients with pre-existing comorbidities demonstrated significantly higher rates of hospital-acquired infections (p < 0.05).
Conclusion
Multidrug-resistant isolates are more prevalent in HAIs than in community-acquired infections, highlighting the need for enhanced surveillance to optimize antibiotic use and control HAIs through early detection of resistance.
Introduction
Hospitals represent a potentially hazardous environment due to various virulent pathogens introduced by admitted patients from the community. These patients are subsequently exposed not only to the hospital's endemic flora but also to microorganisms carried by other ill individuals [1]. This occurs due to a compromised immune defense and colonization by resistant organisms [2]. Hospital-acquired infections (HAIs) are a frequent occurrence in healthcare facilities globally, with their prevalence exceptionally high in resource-limited developing countries [3]. The extensive use of broad-spectrum antibiotics in hospitals creates an intense selective pressure, fostering the emergence of antibiotic-resistant bacteria and complicating the treatment of these infections. As a result, HAIs have been recognized as a severe public health issue for over a century, contributing to poor health outcomes and significantly impacting the quality of healthcare delivery [4].
Hospital-acquired infections most commonly manifest as urinary tract infections, respiratory tract infections, circulatory system infections, and surgical site infections [5]. A World Health Organization report covering 55 hospitals across 14 countries found that 8.7% of hospitalized patients developed HAIs, with the highest prevalence observed in the Eastern Mediterranean Region and lower rates in the Western Pacific [5]. The prevalence of HAIs has been reported at approximately 5% in North America and parts of Europe while reaching up to 40% in some areas of Asia, Latin America, and Africa [6]. A European study reported the prevalence of HAIs to be approximately 2.9%. Several factors contribute to the occurrence of HAIs, including medical interventions, substandard hospital environments, and inadequate personal hygiene practices among both hospital staff and patients [7]. However, the primary driver of HAIs is the failure to adhere to health and safety protocols in healthcare settings. While it is impossible to eliminate HAIs, even in highly advanced hospitals, strict adherence to established standards and guidelines can significantly reduce or manage their occurrence, especially in regions such as Africa [6]. In modern healthcare, where technological advancements and high expectations for quality care prevail, it is critical to thoroughly examine the frequency and underlying causes of HAIs. The absence of accurate data on the prevalence of HAIs poses significant challenges to executing these control measures, leading to increased healthcare costs for both health systems and patients [8].
Despite the global recognition of these pathogens, limited studies have compared microorganisms from both community and hospital settings; therefore, the current study aims to fill this gap by comparing microorganisms isolated from community-acquired and HAIs. It also seeks to explore the relationship between these infections and patients' comorbidities and socio-demographic factors.
Methods
Study design and setting
This retrospective cross-sectional study was conducted at Smart Health Tower, Iraq, between January 2023 and December 2023. It included patients from various departments of the hospital, with infections categorized as either community-acquired or HAIs. The Kscien Organization approved the study for Ethical Approval, reference number 24/No. 27, ensuring all ethical guidelines were followed throughout the study.
Sample collection and study population
Data were meticulously extracted from the records of patients who had their samples processed in the microbiology laboratory. Inclusion criteria encompassed all available clinical samples, including blood cultures, urine samples, sputum and bronchoalveolar lavage, wound swabs, and other body fluids. Patients were classified into either the CAI or HAI group based on guidelines from the Centers for Disease Control and Prevention and the Infectious Diseases Society of America. The CAIs were defined as infections present at the time of hospital admission or within 48 hours of admission, with no history of recent healthcare exposure, such as hospitalization within the previous 90 days. In contrast, HAIs were defined as infections that developed 48 hours or more after hospital admission and were associated with invasive procedures or prior healthcare exposure [9]. Patients with incomplete data were excluded to ensure the accuracy and reliability of the study findings.
Bacterial identification
Bacterial identification was conducted using conventional methods and the BD Phoenix™ M50 automated identification and susceptibility testing system, specifically tailored to the diverse range of clinical samples processed during the study. Blood cultures were incubated in the BD BACTEC™ automated blood culture system, following established protocols, for up to five days to detect the growth of bacteria or fungi, with positive cultures subsequently sub-cultured onto solid media, including blood agar and chocolate agar, to enhance isolation of pathogens. Urine samples were plated on cystine lactose electrolyte-deficient agar and MacConkey agar to promote the growth of Escherichia coli, Klebsiella, and other common uropathogens. Body fluids were inoculated onto blood and chocolate agar. To identify respiratory pathogens, sputum samples were Gram-stained and cultured on selective media, including MacConkey and blood agar. Wound swabs were processed on blood agar and mannitol salt agar. The BD Phoenix™ M50 system was utilized for precise species-level identification and antimicrobial susceptibility testing, providing comprehensive biochemical profiles for various pathogens [10]. This combination of conventional and automated methods ensured accurate identification and susceptibility testing across all clinical sample types, adhering to CLSI (Clinical and Laboratory Standards Institute) guidelines for bacteriological analysis [11]. For samples that did not exhibit visible growth after the initial 24 hours, the incubation was extended to 48 hours.
Antibiotic susceptibility testing
Antimicrobial susceptibility testing was conducted using two methods. The classical Kirby-Bauer disk diffusion method was performed according to Clinical and Laboratory Standards Institute guidelines [11], where standardized antibiotic disks were applied to Mueller-Hinton agar plates inoculated with bacterial suspensions, and inhibition zone diameters were measured and interpreted using CLSI breakpoints (Figure 1). Additionally, the BD Phoenix™ M50 automated system was used to confirm susceptibility results and to test a broader range of antimicrobials, providing Minimum Inhibitory Concentration (MIC) values and classifying isolates as susceptible, intermediate, or resistant based on CLSI interpretive criteria. The antibiotics tested included Amikacin, Gentamicin, Gentamicin-Syn, Ampicillin-sulbactam, Ampicillin, Amoxicillin, Amoxicillin-Clavulanate, Piperacillin-Tazobactam, Piperacillin, Penicillin G, Oxacillin, Cefuroxime, Ceftriaxone, Cefepime, Cefoxitin, Ceftaroline, Cefpodoxime, Cefixime, Cefotaxime, Clarithromycin, Azithromycin, Erythromycin, Ciprofloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, Ofloxacin, Trimethoprim-Sulfamethoxazole, Vancomycin, Teicoplanin, Daptomycin, Clindamycin, Tetracycline, Doxycycline, Minocycline, Tigecycline, Imipenem, Meropenem, Nitrofurantoin, Linezolid, Rifampin, Chloramphenicol, Mupirocin High level. This combined approach ensured consistent and accurate interpretation of susceptibility results, enhancing the reliability of the findings.
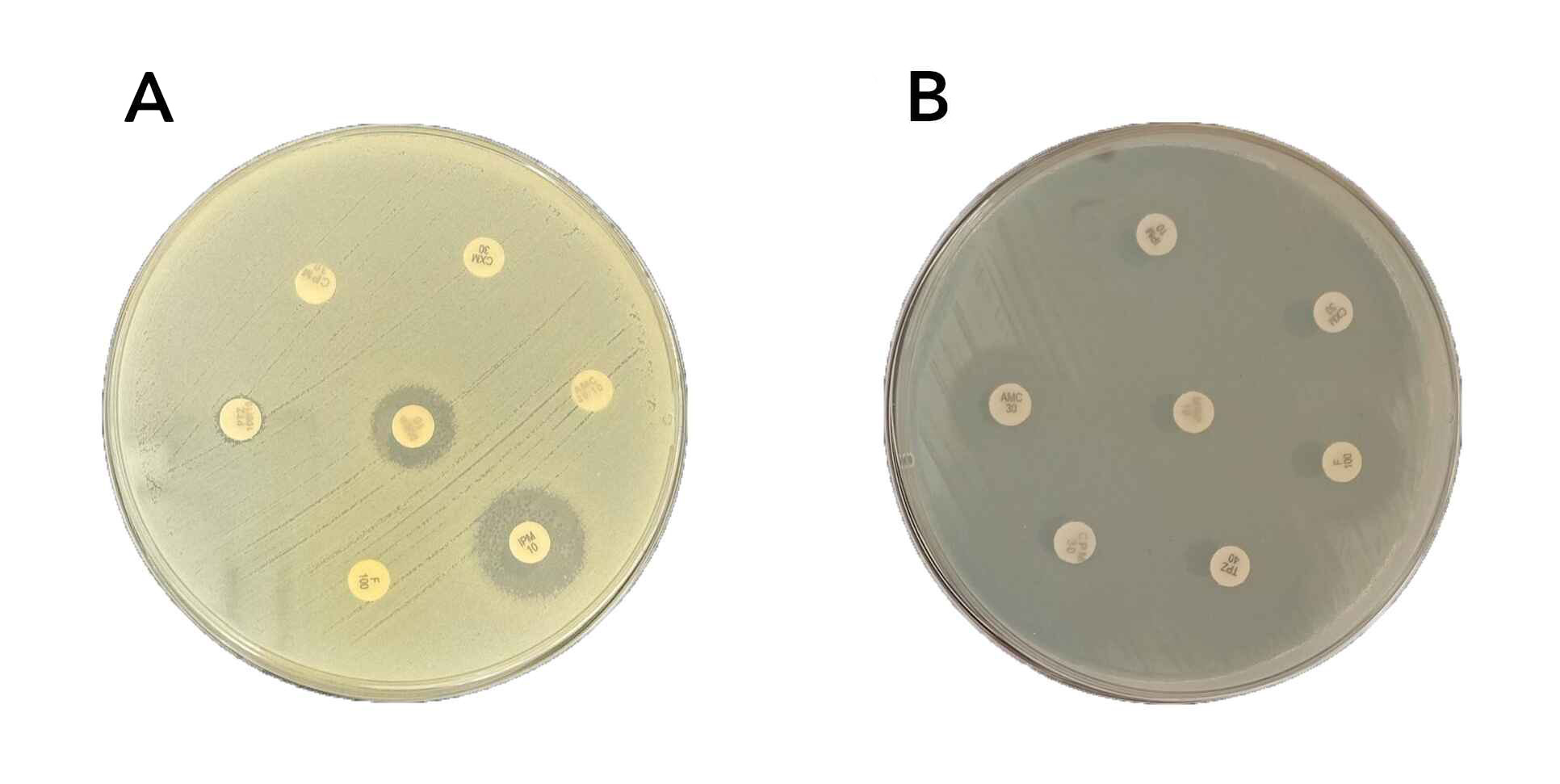
Antibiotic classification and multidrug resistance
The antibiotics were categorized into seven groups: aminoglycosides, beta-lactams, macrolides, sulfonamides, tetracyclines, glycopeptides, and fluoroquinolones. Multidrug-resistant (MDR) isolates were defined as bacterial strains resistant to three or more of these antibiotic classes, following established criteria [12]. This classification facilitated a comprehensive analysis of antimicrobial resistance patterns and enabled the identification of the most challenging cases of antibiotic resistance, providing critical insight into the prevalence of MDR organisms.
Data analysis
Data on bacterial isolates, antimicrobial susceptibility profiles, patient demographics, infection types, and antibiotic resistance patterns were systematically collected and entered into Microsoft Excel 2007 before being transferred to SPSS version 25 for statistical analysis. Statistical evaluations were conducted to assess differences in resistance rates between CAIs and HAIs, stratified by infection site (e.g., bloodstream, urinary tract, respiratory tract) and pathogen type. Descriptive statistics summarized the demographic and clinical characteristics of patients, while resistance rates were compared using Chi-square tests for categorical variables and t-tests for continuous variables. The analysis encompassed calculating prevalence rates, frequencies, susceptibility patterns, and other descriptive statistics, with statistical significance set at a p-value of equal to or less than 0.05 for the chi-square test, which compared categorical variables with bacterial growth.
Results
Microbial growth and participant characteristics
In this study involving 2157 participants, 1303 (60.4%) were male. Microbial growth was observed in 1177 cases (54.6%). Notably, 535 (41.1%) of the females and 445 (52.1%) of the males exhibited no growth, indicating a significant difference (p < 0.001). The mean age of participants was 43.62± 23.3years. The highest growth rate was observed in wound samples (187, 91.2%), while body fluids showed the lowest rate (171, 33.0%), reflecting a statistically significant difference (p < 0.001). The sample collection location did not significantly influence growth, with no growth in 475 (44.7%) from community settings and 216 (46.7%) from hospitals (p = 0.502). Among the various comorbidities, obesity, renal insufficiency, and diabetes, significantly differed between participants with microbial growth and those without growth(P<0.05) (Table 1).
|
Variables |
Bacterial Growth |
Total |
P-Value |
|
|
No Growth |
Growth |
|||
|
Gender (N, %) Female Male |
535(41.1) 445(52.1) |
768(58.9) 409(47.9) |
1303 (100) 854 (100) |
|
|
Age (Year, Mean± SD) |
43.38± 23.5 |
43.83± 23.2 |
43.62± 23.3 |
0.653 |
|
Type of clinical sample (N, %) Urine Body fluids Respiratory samples Wound Stool Pus Others |
534(45.1) 347(67.0) 32(35.6) 18(8.8) 8(42.1) 8(30.8) 33(28.9) |
651(54.9) 171(33.0) 58(64.4) 187(91.2) 11(57.9) 18(69.2) 81(71.1) |
1185(100) 518(100) 90(100) 205(100) 19(100) 26(100) 114(100) |
<0.001 |
|
Setting (N, %) Community Hospital Not mentioned |
216(46.7) 289(55.0) |
587(55.3) 247(53.3) 343(45.0) |
1062(100) 463(100) 632(100) |
0.502 |
|
Length of hospital stay (Day, Mean± SD) |
12.76± 27.72 |
9.42± 19.96 |
10.99± 23.95 |
0.137 |
|
Asthma (N, %) Yes No Not mentioned |
664(44.8) 290(46.2) |
22(45.8) 817(55.2) 338(53.8) |
48(100) 1482(100) 628(100) |
0.400 |
|
Pregnancy (N, %) Yes No Not mentioned |
24(43.6) 650(46.0) 306(44.5) |
31(53.4) 764(54.0) 382(55.5) |
55(100) 1414(100) 625(100) |
0.783 |
|
Heart Failure (N, %) Yes No Not mentioned |
96(49.2) 597(44.7) 287(45.9) |
99(50.8) 740(55.3) 338(54.1) |
195(100) 1337(100) 625(100) |
0.467 |
|
Renal insufficiency (N, %) Yes No Not mentioned |
67(35.4) 626(46.6) 287(45.9) |
122(64.6) 717(53.4) 338(54.1) |
189(100) 1343(100) 625(100) |
0.015 |
|
Hypertension (N, %) Yes No Not mentioned |
139(41.9) 554(46.2) 287(45.9) |
193(58.1) 646(53.8) 338(54.1) |
332(100) 1200(100) 625(100) |
0.364 |
|
Obesity (N, %) Yes No Not mentioned |
101(37.3) 591(46.9) 288(46.0) |
170(62.7) 669 (53.1) 338(54.0) |
271(100) 1260(100) 626(100) |
0.014 |
|
Malignant (N, %) No Not mentioned |
52(41.6) 641(45.6) 287(45.9) |
73(58.4) 766(54.4) 338(54.1) |
125(100) 1407(100) 625(100) |
0.667 |
|
Diabetes (N, %) Yes No Not mentioned |
103(35.4) 590(47.5) 287(46.0) |
188(64.6) 652(52.5) 337(54.0) |
291(100) 1242(100) 624(100) |
0.001 |
Distribution of isolated bacteria by setting
In this study, among the 449-gram negative bacterial isolates, 301 (67.0%) were from community settings, and 148 (33.0%) were from hospitals. Escherichia coli was the most prevalent, with 245 isolates, 179 (73.1%) from community settings and 66 (26.9%) from hospitals. Other notable gram-negative bacteria included Klebsiella pneumonia (64 isolates; 62.5% community vs. 37.5% hospital) and Pseudomonas aeruginosa (42 isolates; 50% each from community and hospital). The gram-positive bacteria primarily included Streptococcus species (100 isolates; 83(83.0%) community vs. 17(17.0%) hospital) and Enterococcus faecalis (72 isolates; 58(80.6%) community vs. 14(19.4%) hospital). Overall, gram-positive bacteria comprised 149 isolates, with a higher occurrence in community settings 284(75.3%) compared to hospitals 93(24.7%) (Table 2).
|
Gram-Positive/Negative |
Microorganism N (%) |
Source of Infection | Total | |
| Community | Hospital | |||
| Gram Negative |
Escherichia coli |
179(73.1) |
66(26.9) |
245(100.0) |
|
Klebsiella pneumonia |
40(62.5) |
24(37.5) |
64(100.0) |
|
|
Pseudomonas aeruginosa |
21(50.0) |
21(50.0) |
42(100.0) |
|
|
Proteus species |
12(70.6) |
5(29.4) |
17(100.0) |
|
|
Morganella morganii |
7(77.8) |
2(22.2) |
9(100.0) |
|
|
Citrobacter species |
7(87.5) |
1(12.5) |
8(100.0) |
|
|
Achromobacter spp. |
3(37.5) |
5(62.5) |
8(100.0) |
|
|
Moraxella species |
5(62.5) |
3(37.5) |
8(100.0) |
|
|
Klebsiella species |
4(57.1) |
3(42.9) |
7(100.0) |
|
|
Serratia species |
4(66.7) |
2(33.3) |
6(100.0) |
|
|
Salmonella species |
4(80.0) |
1(20.0) |
5(100.0) |
|
|
Enterobacter species |
4(80.0) |
1(20.0) |
5(100.0) |
|
|
Burkholderia cepacia |
1(20.0) |
4(80.0) |
5(100.0) |
|
|
Acinetobacter species |
1(25.0) |
3(75.0) |
4(100.0) |
|
|
Cedecea davisae |
2(100.0) |
0(0.0) |
2(100.0) |
|
|
Pasteurella multocida |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Pseudomonas aeruginosa |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Alloiococcus otitidis |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Arcanobacterium species |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Alcaligenes faecalis |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Pasteurella multocida |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Providencia rettgeri |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Vibrio vulnificus |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Pantoea agglomerans |
2(100.0) |
0(0.0) |
2(100.0) |
|
|
Pseudomonas species |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Rhizobium radiobacter |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Stenotrophomonas maltophilia |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Tatumella ptyseos |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Total |
301(67.0) |
148(33.0) |
449(100.0) |
|
| Gram Positive |
Streptococcus species |
83(83.0) |
17(17.0) |
100(100.0) |
|
Enterococcus faecalis |
58(80.6) |
14(19.4) |
72(100.0) |
|
|
Staphylococcus haemolyticus |
46(79.3) |
12(20.7) |
58(100.0) |
|
|
Staphylococcus epidermidis |
31(64.6) |
17(35.4) |
48(100.0) |
|
|
Staphylococcus aureus |
27(57.4) |
20(42.6) |
47(100.0) |
|
|
Staphylococcus species |
15(71.4) |
6(28.6) |
21(100.0) |
|
|
Corynebacterium species |
9(69.2) |
4(30.8) |
13(100.0) |
|
|
Arcanobacterium species |
4(100.0) |
0(0.0) |
4(100.0) |
|
|
Lactobacillus species |
3(100.0) |
0(0.0) |
3(100.0) |
|
|
Pediococcus pentosaceus |
0(0.0) |
2(100.0) |
2(100.0) |
|
|
Micrococcus lylae |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Alloiococcus otitidis |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Bacillus circulans |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Gemella morbillorum |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Kytococcus sedentarius |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Kocuria Kristinae |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Rothia dentocariosa |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Sreptococcus species |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Total |
284(75.3) |
93(24.7) |
377(100.0) |
|
Antibiotic sensitivity and resistance in community setting
In community settings, among the tested gram-positive isolates, the highest sensitivity rates were observed for imipenem 95(96.9%), followed closely by linezolid at 151(95.6%), meropenem at 96 isolates (94.1%), tigecycline at 61 isolates (93.9%), and daptomycin at 59 isolates (93.7%). Conversely, the highest antibiotic resistance rates were recorded for azithromycin 19(90.5%), followed by ofloxacin 19 isolates (76.0%), and cefixime 68 isolates (74.7%). The overall resistance rate among gram-positive isolates was 1732 (38.7%). For gram-negative isolates, sensitivity rates were as follows: meropenem at 260 isolates (94.5%), tigecycline at 61(93.9%), imipenem at 225(85.2%), and amikacin at 128(81.5%). Notably, high resistance rates were seen, with 20 isolates (100.0%) resistant to clindamycin and 119 isolates (92.2%) resistant to ampicillin. The overall resistance rate among gram-negative isolates was 1614 (37.7%) (Suppl 1).
Antibiotic sensitivity and resistance in hospital isolates
In hospital settings, gram-positive isolates exhibited the highest sensitivity to daptomycin (43 isolates, 93.5%), followed by linezolid (62 isolates, 92.5%), teicoplanin (54 isolates, 91.5%), and tigecycline (37 isolates, 90.2%). The most significant resistance rates were observed for azithromycin (11 isolates, 84.6%) and cefixime (16 isolates, 80.0%). The overall antibiotic resistance rate among gram-positive isolates was 727 (41.1%). For gram-negative isolates, the highest sensitivity rates were noted for meropenem (108 isolates, 85.0%), imipenem (103 isolates, 79.8%), amikacin (80 isolates, 73.4%), and piperacillin-tazobactam (88 isolates, 71.5%). However, resistance was notably high for ampicillin (84 isolates, 95.6%) and cefazolin (80 isolates, 85.1%). The overall resistance rate among gram-negative isolates was 1044 (50.8%) (Suppl 2).
MDR rates in community-acquired infections
In the community setting, MDR among gram-negative bacterial isolates was observed in 183 cases (63.1%). Notably, all Morganella morganii isolates (7, 100.0%) and 3(75.0%) of Klebsiella species and Salmonella species were classified as MDR. Among gram-positive isolates, MDR was present in 171 cases (61.9%), with Lactobacillus species showing 100.0% MDR (3 isolates) and Staphylococcus aureus exhibiting a high MDR rate, with 21 out of 27 isolates (77.8%). Overall, the MDR rate in community-acquired infections was 62.5% (Suppl 3).
MDR rates in hospital-acquired infections
In the hospital setting, MDR was observed in 113 gram-negative bacterial isolates (86.2%). Notably, all isolates of Proteus species, Burkholderia cepacia, and Achromobacter species (100%) were classified as MDR. Among gram-positive isolates, 59 cases (68.6%) exhibited MDR, with Staphylococcus haemolyticus showing an MDR rate of 83.3% (10 out of 12 isolates) and Enterococcus faecalis at 78.6% (11 out of 14 isolates). Overall, the MDR rate in hospital-acquired infections was 79.3% (Suppl 3).
Risk factors for community vs. hospital-acquired infections
In the analysis of risk factors for community-acquired versus hospital-acquired infections, males had a significantly higher proportion of hospital-acquired infections, with 688 (75.4%) compared to 374 (61.0%) in community-acquired infections (p<0.001). Individuals over 40 years old were more likely to have hospital-acquired infections, 280 (35.2%) versus 183(25.1%) in the community-acquired group (p<0.001). Patients with pre-existing comorbidities, including diabetes, malignancy, obesity, hypertension, renal insufficiency, heart failure, and asthma, demonstrated significantly higher rates of hospital-acquired infections (p < 0.05) (Table 3).
| Risk Factors | Infection Source | P-Value | |
|
Community acquired |
Hospital acquired |
||
|
Gender (N, %) Male Female |
688(75.4) |
224(24.6) |
<0.001 |
|
Age <40 |
516(64.8) |
280(35.2) |
<0.001 |
|
Diabetes Yes No |
903(73.2) |
331(26.8) |
<0.001 |
|
Malignancy Yes No |
1005(71.8) |
395(28.2) |
|
|
Obesity Yes No |
890(71.0) |
364(29.0) |
0.034 |
|
Hypertension Yes No |
877(73.5) |
316(26.5) |
<0.001 |
|
Renal Insufficiency Yes No |
961(71.9) |
375(28.1) |
<0.001 |
|
Heart Failure Yes |
966(72.6) |
364(27.4) |
<0.001 |
|
Pregnancy Yes No |
1010(68.7) |
460(31.3) |
<0.001 |
|
Asthma Yes No |
1040(70.4) |
437(29.6) |
0.001 |
Discussion
Antimicrobial resistance (AMR) has become one of the most critical global public health challenges of the 21st century. It arises when microorganisms resist antimicrobial drugs such as antibiotics, rendering these treatments ineffective. This resistance primarily results from the overuse and misuse of antibiotics in various sectors, including clinical settings. Often referred to as the "Silent Pandemic," AMR demands immediate and effective action rather than being treated as a distant concern [13]. Despite the growing threat of antimicrobial resistance, the overuse of these agents remains prevalent, particularly in patients with critical illnesses, advanced disease stages, malignancies, or immunocompromised conditions [14].
Hospitals are recognized as high-risk environments for health, particularly due to the prevalence of HAIs in both developed and developing countries [15]. The impact of HAIs is substantial, contributing to increased healthcare costs, greater disease severity, higher rates of antimicrobial resistance, and elevated morbidity and mortality. Within healthcare settings, bacterial pathogens are the primary culprits behind nosocomial infections, with many strains exhibiting resistance to both standard and last-resort antibiotics [16].
Gram-negative bacteria are frequently involved in HAIs, accounting for up to 87% of cases [15]. Among Gram-positive bacteria, Staphylococcus aureus is the most prevalent strain [17]. In Europe and Asia, the most common Gram-negative pathogens include Pseudomonas aeruginosa, Acinetobacter baumannii, and members of the Enterobacteriaceae family [18,19]. A multicenter retrospective study conducted across five private hospitals in Lebanon, involving 258 patients, reported that Escherichia coli and Pseudomonas aeruginosa were the most prevalent Gram-negative bacteria, while Staphylococcus aureus was the dominant Gram-positive isolate [1]. Similarly, the present study found that Gram-negative bacteria accounted for 62.1% (148 out of 241) of hospital-acquired infections (HAIs). The most frequently isolated Gram-negative pathogens were Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Among Gram-positive bacteria, Staphylococcus aureus emerged as the most prevalent strain in the hospital setting.
Hospital-acquired microorganisms exhibited greater resistance to antimicrobials than their community-acquired counterparts. For instance, a study by Matta et al. demonstrated that hospital-acquired Pseudomonas aeruginosa showed significantly higher resistance to all evaluated antimicrobial agents thanacquired strains [1]. In the current study, the resistance rate among community-acquired infections was 38.2% (3,346 out of 8,760 isolates), whereas the resistance rate among hospital-acquired infections was 46.3% (1,771 out of 3,825 isolates).
Escherichia coli infections are typically treated with antibiotics such as ciprofloxacin, levofloxacin, and other fluoroquinolones; however, resistance to multiple antibiotics has become increasingly prevalent. The growing resistance to fluoroquinolones and the emergence of extended-spectrum beta-lactamases pose significant challenges in managing these infections. Although carbapenems are generally considered the preferred treatment for MDR Escherichia coli infections, reports of resistance to carbapenems are also rising [15]. In this study, sensitivity rates for Escherichia coli isolates in community-acquired infections were found to be 49.4%, 53%, and 60.3% for ciprofloxacin, levofloxacin, and norfloxacin, respectively. In contrast, sensitivity rates among hospital-acquired isolates were lower, with 29.7%, 33.3%, and 28.6% for the same antibiotics. Furthermore, sensitivity to imipenem and meropenem was observed in 91.9% and 96.0% of community-acquired Escherichia coli isolates, while sensitivity in hospital-acquired cases was notably lower at 83.3% and 87.9%. These findings indicate a concerning trend of increased antibiotic resistance among Escherichia coli isolates from hospital settings, particularly concerning carbapenem resistance.
Klebsiella pneumoniae is the second most prevalent cause of HAIs, following Escherichia coli [15]. While it is primarily considered an opportunistic pathogen, there has been a notable increase in its hypervirulence, often linked to hypercapsulation [20], along with a rise in antibiotic resistance [21]. The emergence of carbapenem-resistant Klebsiella pneumoniae strains poses a significant global health threat, contributing to increased mortality rates primarily due to the acquisition of Klebsiella pneumoniae carbapenemases [22]. Multidrug-resistant strains can exhibit resistance to all beta-lactams and fluoroquinolones. Consequently, last-resort treatment options often involve polymyxin B, frequently in combination with tigecycline or certain aminoglycosides [15]. In this study, community-acquired Klebsiella pneumoniae isolates showed a sensitivity rate of 100% to tigecycline, whereas the sensitivity among hospital-acquired isolates was significantly lower at 68.4%. Additionally, fewer than 50% of K. pneumoniae isolates demonstrated sensitivity to all beta-lactam antibiotics.
A study conducted in India investigating the etiology and antimicrobial sensitivity of organisms responsible for community-acquired pneumonia, which included 145 patients, found Streptococcus infections to be the most frequently isolated pathogen in the community setting [23]. In line with these findings, the current study also identified Streptococcus infections as one of the most commonly isolated pathogens within the community context. This could be explained by high transmissibility, opportunistic nature in vulnerable populations, association with diverse infections, seasonal peaks, and the dynamics of antimicrobial resistance and vaccination.
In recent decades, the prevalence of antimicrobial resistance has escalated worldwide, with MDR bacteria emerging as a significant cause of nosocomial infections. The risk of MDR infections is linked to several factors, including prolonged antimicrobial therapy, cross-transmission, extended hospital stays, and invasive procedures. These resistant bacteria can lead to various infections—such as pneumonia, urinary tract infections, and wound infections—associated with increased morbidity, and mortality [24]. In this study, a higher MDR was found among HAIs compared to Community acquired infections, with 79.3% for HAI and 62.5% for Community acquired setting. Higher rates of MDR in HAIs compared to community-acquired infections result from factors such as prolonged antibiotic use, invasive procedures, and close patient proximity, which foster the emergence and spread of resistant strains. A retrospective study conducted in a tertiary general hospital in Jining, China, revealed a high prevalence of MDR HAIs; out of 7,579 bacterial isolates, 3,223 (42.5%) were identified as MDR. Gram-negative bacteria were the most frequently isolated MDR pathogens, with Escherichia coli exhibiting the highest detection rate at 37.7%. Collectively, Escherichia coli and Klebsiella pneumoniae accounted for 51.0% of all MDR isolates [24]. In this study, the prevalence of MDR among hospital settings was found to be 79.3%, with 172 out of 217 isolates classified as MDR. Consistent with previous findings, gram-negative bacteria were the most frequently isolated MDR pathogens, with Escherichia coli detected in 24.9% (54 out of 217) of cases, followed by Klebsiella pneumoniae at 10.1% (22 out of 217).
Multidrug-resistant Staphylococcus aureus is a leading cause of HAIs and a significant contributor to mortality among hospitalized patients, largely due to its possession of resistance genes against various antibiotics, including commonly used anti-staphylococcal drugs [25]. In this study, 25.4% (15 out of 59) of the MDR Gram-positive isolates from hospital settings were identified as multidrug-resistant Staphylococcus aureus. Most Staphylococcus aureus isolates exhibited resistance to penicillin, while all were sensitive to the carbapenems.
A prospective cohort study conducted over one year at a university tertiary care hospital in Portugal identified neoplastic diseases, including hematologic malignancies and solid tumors, as well as immunocompromised states, as common conditions associated with hospital-acquired infections [26]. Notably, no gender differences were observed in infection rates [1]. In this study, patients with pre-existing comorbidities such as diabetes, malignancy, obesity, hypertension, renal insufficiency, heart failure, and asthma were found to have significantly higher rates of hospital-acquired infections.
Conclusion
Multidrug-resistant infections were prevalent in HAIs, with most isolates resistant to current antibiotics. This underscores the need for enhanced surveillance to optimize antibiotic use and control HAIs. The higher resistance in HAIs compared to community-acquired infections highlights the importance of early detection of resistance.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The study's ethical approval was obtained from the scientific committee of the Kscien Organization for Scientific Research.
Patient consent (participation and publication): Verbal informed consent was obtained from patients for participation in this study and publication.
Source of Funding: Saaeda company.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: BAA and FHK were significant contributors to the conception of the study and the literature search for related studies. RQS, HAY, WAH and SHK were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. AMM, FA, and KKM were involved in the literature review, study design, and manuscript writing. DQM, BHI, HSA, SHA, MOS and SSA Literature review, final approval of the manuscript, and processing of the tables. RQS and AMM confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.

Phenotypic and Molecular Characterization of the blaTEM Gene in Extended-Spectrum Beta-Lactamase-Producing Klebsiella pneumoniae
Marwan Arkan Ghafoor, Kasya Aswad Othman, Lanja Jalal Mahmood, Laali Khan Hamad Jabbar, Kashma...
Abstract
Introduction
There has been a notable rise in antibiotic resistance among enterobacteria. This issue is primarily attributed to the emergence of extended-spectrum beta-lactamases (ESBLs), which present a significant concern for public health worldwide. This study investigates the prevalence of ESBL production, antibiotic resistance profiles, and molecular identification of the blaTEM gene in Klebsiella pneumoniae isolates.
Methods
The samples were randomly collected from several medical facilities in Erbil city. The VITEK 2 system was used for bacterial identification, antibiotic susceptibility, and ESBL production testing. The Double Disc Synergy Test (DDST) confirmed ESBL production. Polymerase chain reaction was conducted on all DNA samples, and the amplified DNA was analyzed using agarose gel electrophoresis to detect the blaTEM gene.
Results
A total of 43 samples were collected, of which the majority were urine (56%), followed by sputum (28%), blood (9%), and wound (7%). Klebsiella pneumoniae isolates exhibited the highest prevalence of resistance against ceftazidime (72%), ceftriaxone (70%), ciprofloxacin (63%), amoxicillin-clavulanic acid (60%), amikacin (58%), cefotaxime/tazobactam (56%), and gentamicin (53%). The DDST results indicated positive ESBL production in 15 isolates (35%), as evidenced by an increase or distortion in the inhibition zone toward the amoxicillin-clavulanate disc. Of the 43 isolates, 34 (79%) carried the blaTEM gene.
Conclusion
The study area shows a significant level of antibiotic resistance in ESBL-producing Klebsiella pneumoniae isolates, which, if not adequately addressed, could soon lead to severe health and therapeutic consequences.
Introduction
Antibiotic resistance is a dynamic and ongoing challenge. Over the past three decades, there has been a notable rise in antibiotic resistance among enterobacteria, particularly concerning third-generation cephalosporins. This issue is primarily attributed to the emergence of extended-spectrum beta-lactamases (ESBLs). ESBLs are a group of beta-lactamase enzymes produced by gram-negative bacteria that can hydrolyze and inactivate many antibiotics, including aztreonam, penicillin, and cephalosporins. These enzymes, such as TEM, SHV, and CTX-M, and their variants, allow enterobacteria to resist β-lactam antibiotics. The encroachment of these multi-drug-resistant pathogens into community settings raises significant alarm. The swift spread of the genes that encode these ESBLs, primarily via plasmids, has facilitated their rapid proliferation, leading to an alarming increase in the global prevalence of ESBL-producing bacteria. This situation presents a significant concern for public health worldwide [1,2]. Klebsiella pneumoniae is an opportunistic pathogen responsible for nosocomial and community-acquired infections [3]. This bacterium is usually found as an intestinal microflora. It can cause severe diseases like ventilator-associated pneumonia, catheter-related urinary tract infections, meningitis, bacteremia, septicemia, infection of surgical and non-surgical wounds, diarrhea, prosthetic valve endocarditis, peritonitis, and osteomyelitis [1]. The first strains of Klebsiella pneumoniae with ESBL (KP-ESBL) were identified in Europe in 1982, marking the emergence of resistance to ceftazidime and aztreonam due to plasmid-mediated beta-lactamase enzymes. These resistance traits rapidly disseminated to other gram-negative bacteria, including Escherichia coli. Since the discovery of these enzymes, their prevalence has continued to rise, with over 200 distinct ESBL enzymes identified today. The impact of ESBL-producing strains is particularly pronounced in intensive care units, which pose a high risk for epidemic outbreaks. K. pneumoniae and E. coli are the two most frequently encountered bacterial species associated with ESBL production. Implementing proactive monitoring of ESBL-producing pathogens in high-risk populations is crucial through suitable antimicrobial strategies. This necessity arises from the tendency of these pathogens to display multidrug resistance, which complicates treatment options and increases the potential for severe infections [4]. This study investigates the prevalence of ESBL production, antibiotic resistance profiles, and molecular identification of the blaTEM gene in Klebsiella pneumoniae isolates in Erbil City.
Methods
Study design and sample collection
This retrospective cross-sectional study was conducted between September 2021 and January 2022. The ethics board of the College of Health Sciences at Hewler Medical University approved the study. Patient consent was verbally obtained from the patients to collect their samples and be published in this study. The samples were randomly collected from several medical facilities in Erbil city, such as Nanakali Hospital, Hawler Teaching Hospital, and private laboratories. The collected samples included urine, sputum, or wound infection.
Isolation, identification, and susceptibility test of Klebsiella pneumoniae
The samples were cultured on MacConkey agar and incubated at 37°C for 24 hours. Klebsiella pneumoniae colonies were isolated based on their large, pink-to-red mucoid appearance. The VITEK 2 system (bioMérieux, France) was used for bacterial identification and antibiotic susceptibility testing. A sterile swab or stick was used to transfer an adequate number of colonies from the cultured sample into 3.0 ml of sterile saline (0.45% to 0.50% NaCl, pH 4.5 to 7) in a 12 × 75 mm polystyrene test tube. The suspension’s turbidity was then adjusted to match the 0.5 McFarland standard for antimicrobial susceptibility testing and measured using a DensiChek™ turbidimeter.
Phenotypic Detection and Confirmation of ESBLs
ESBL production was detected among the isolates using the VITEK 2 system. The testing panel included six wells containing 10 μg/ml ceftriaxone, 0.5 μg/ml cefotaxime, or 0.5 μg/ml ceftazidime, alone or in combination with clavulanic acid (10 μg/ml, 4 μg/ml, and 4 μg/ml, respectively). Bacterial growth in each well was quantitatively assessed using an optical scanner. A proportional reduction in growth in the wells containing cephalosporin combined with clavulanic acid, compared to those containing cephalosporin alone, was considered indicative of ESBL production. The Double Disc Synergy Test (DDST) confirmed ESBL production. Each Klebsiella pneumoniae isolate was inoculated on Müller-Hinton agar plates for susceptibility testing. Discs containing 30 μg of cefotaxime and ceftazidime were placed on either side of a disc with co-amoxiclav (20/10 μg), positioned 20 mm apart (center to center). ESBL production was indicated when the inhibition zone of either cephalosporin was expanded by clavulanic acid, often resulting in a distinctive "champagne-cork" or "keyhole" shape zone [1].
Molecular detection of blaTEM gene
DNA extraction of Klebsiella pneumoniae was carried out using the simple boiling method. Isolated colonies from overnight cultures were placed in a test tube containing 1 ml of distilled water and heated in a water bath for 10 minutes. The sample was centrifuged at 1,000 rpm for 5 minutes to complete the extraction process. Polymerase chain reaction (PCR) was conducted on all DNA samples of Klebsiella pneumoniae. The concentration of extracted double-stranded DNA was combined with a PCR master mix (Promega, USA), which included Taq DNA polymerase, deoxynucleotide triphosphates (dATP, dCTP, dGTP, dTTP), buffers, and salts. Specific forward (5'-GAG TAT CAA CAT TTC CGT GTC-3') and reverse (5'-TAA TCA GTG GGC ACC TTC TC-3') primers were added to amplify the blaTEM target gene. The PCR mixture was then incubated in a thermocycler, undergoing 32 cycles of alternating temperatures: a denaturation step at 94°C for one minute to separate the double-stranded DNA into single strands, an annealing step at 57°C for one minute to allow the primers to bind to the target DNA, and an extension step at 72°C for 10 minutes for the Taq DNA polymerase to synthesize new DNA strands. This process generated billions of copies of the target DNA sequence within a few hours. Subsequently, the amplified DNA was separated and analyzed using agarose gel electrophoresis.
Results
A total of 43 samples were collected; 22 (51%) were obtained from females and 21 (49%) from males. The majority of the samples were urine (56%), followed by sputum (28%), blood (9%), and wound (7%). Klebsiella pneumoniae isolates exhibited the highest prevalence of resistance against ceftazidime (72%), ceftriaxone (70%), ciprofloxacin (63%), amoxicillin-clavulanic acid (60%), amikacin (58%), cefotaxime/tazobactam (56%), and gentamicin (53%). In contrast, the lowest prevalence of resistance was observed with imipenem (26%) and meropenem (28%) (Table 1). The DDST results indicated positive ESBL production in 15 isolates (35%), as evidenced by an increase or distortion in the inhibition zone toward the amoxicillin-clavulanate disc. The remaining samples (65%) tested negative for ESBL production (Figure 1). Of the 43 isolates, 34 (79%) carried the blaTEM gene (Figure 2). Overall, 11 isolates (26%) tested positive in the DDST and PCR, while five isolates (12%) were negative. Additionally, four isolates (9%) were positive in DDST but negative in PCR, whereas 23 isolates (53%) were negative in DDST but positive in PCR (Table 2).

|
Antibiotic |
No. of resistant isolates |
Frequency (%) |
No. of sensitive isolates |
Frequency (%) |
|
Ceftazidime |
31 |
72% |
12 |
28% |
|
Ceftriaxone |
30 |
70% |
13 |
30% |
|
Ciprofloxacin |
27 |
63% |
16 |
37% |
|
Amoxicillin clavulanic acid |
26 |
60% |
17 |
40% |
|
Amikacin |
25 |
58% |
18 |
42% |
|
Cefotaxime |
24 |
56% |
19 |
44% |
|
Tazobactam |
24 |
56% |
19 |
44% |
|
Gentamicin |
23 |
53% |
20 |
47% |
|
Meropenem |
12 |
28% |
31 |
72% |
|
Imipenem |
11 |
26% |
32 |
74% |
| Isolated bacteria |
DDST results |
PCR results |
Number |
Frequency (%) |
|
(+) |
(+) |
11 |
26% |
|
|
(-) |
(-) |
5 |
12% |
|
|
(+) |
(-) |
4 |
9% |
|
|
(-) |
(+) |
23 |
53% |
Discussion
KP-ESBL has emerged as a significant concern due to its virulence factors and role as a leading cause of infectious diseases. It is categorized within the multidrug-resistant group of bacteria. Klebsiella pneumoniae exhibits antibiotic resistance through several mechanisms, including the enzymatic degradation or inactivation of antibiotic compounds, alterations in membrane permeability, and modifications of antibiotic target sites via bacterial protein mutations. It has been reported that K. pneumoniae acquires numerous ESBL enzymes, which serve as its primary defense mechanism against antibiotics [5]. The prompt and precise identification of ESBL-positive Enterobacteriaceae strains is critical for guiding appropriate patient management and developing and enforcing targeted infection control protocols [6]. Over the past decade, numerous studies have highlighted the prevalence of ESBL-mediated resistance in infectious bacteria, with a higher prevalence in Escherichia coli and Klebsiella pneumoniae [2,5,6-9]. In the study by Benbrahim et al., 15 out of 40 (37.5%) Klebsiella pneumoniae isolates were identified as ESBL-producing strains, a figure close to the 41.1% reported by Pirzaman et al. [10]. Another study reported a prevalence of 31.8% [2]. Higher rates were observed in Asian countries, reaching up to 75% [1]. In this study, the prevalence of isolates with positive ESBL enzymes was 35%, close to previously reported [1,2,10].
Infections caused by ESBL-producing bacteria can affect individuals of all ages, though their distribution may be influenced by patients' immunological status and the prevalence of antibiotic misuse. Benbrahim et al. found that KP-ESBLs were detected across all age groups, with the highest incidence (20%) observed in individuals aged 21-30 [1].
Gravey et al. reported a KP-ESBL prevalence of 4.1% in individuals aged 18–64 and 4.2% in those over 65 [11]. Due to the retrospective nature of the present study, the age preference among samples was unknown. In the present study, females comprised 51% of the sample, while males accounted for 49%, a distribution that closely aligns with the findings reported by Marra et al. [12]. However, a study by Ali and Ismael observed a higher proportion of female samples (72.72%) than male samples (22.73%) among 84 Klebsiella pneumoniae isolates [13]. Conversely, a study by Nirwati et al. found a higher frequency of male samples (64.07%) than female samples (35.93%) in 167 K. pneumoniae isolates [14]. In the study by Benbrahim et al., KP-ESBL strains were most frequently isolated from urine samples, which the authors suggested is likely due to urine being one of the most commonly collected specimens for clinical investigation [1]. Similarly, most of the samples (56%) in the present study were urine.
Antimicrobial resistance in pathogenic bacteria presents a global challenge, contributing to high mortality and morbidity rates. Infections caused by multidrug-resistant strains are often difficult or impossible to treat with conventional antimicrobials. The widespread, usually unnecessary use of antibiotics stems from the failure of many healthcare centers to promptly diagnose causative microorganisms and determine their antimicrobial susceptibility in patients with bacteremia and other serious infections [15]. In this study, Klebsiella pneumoniae strains exhibited the highest resistance rates against ceftazidime (72%), ceftriaxone (70%), ciprofloxacin (63%), and amoxicillin-clavulanic acid (60%). The resistance rate for ceftazidime in this study was slightly similar to that reported in another study conducted in Erbil, which documented a resistance rate of 62.5% [16]. Notably, our study found significantly higher resistance rates for ciprofloxacin (63% vs. 4.2%), gentamicin (53% vs. 4.2%), and amikacin (58% vs. 4.2%). Conversely, the resistance rate for amoxicillin-clavulanic acid was lower in our study than in the previous one (60% vs. 81.25%) [16]. Another study conducted in Iran reported even higher resistance rates for amoxicillin-clavulanic acid (100%), ceftazidime (84%), tazobactam (80%), ciprofloxacin (80%), and amikacin (60%) [17]. In contrast to our findings, sensitivity rates of 90% and 100% by K. pneumoniae isolates have been reported for amoxicillin-clavulanic acid and imipenem, respectively [1,18]. Muggeo et al. and Rasamiravaka et al. found a sensitivity rate of 83% to amikacin, while it was 42% in the present study. In the study by Bora et al., imipenem and meropenem were identified as one of the most effective antimicrobial agents against ESBL-producing isolates, comparable to the findings in this study [7].
The prevalence of the blaTEM gene in Klebsiella pneumoniae isolates varies across studies. Alibi et al. reported that blaTEM gene was present in 56.8% of their isolates [19]. In contrast, Paterson et al. found a prevalence of 87% [20]. Guessennd et al. reported that 63.4% of their isolates in Abidjan, Côte d’Ivoire, carried the blaTEM gene [21]. The prevalence rates of 31.57% and 55% for the blaTEM gene in Klebsiella pneumoniae have also been reported [2,5]. Based on genotypic detection, in this study, 79% of the isolates carried the blaTEM gene, which may indicate uncontrolled antibiotic misuse in our locality. In line with this finding, Bora et al. found that genes responsible for ESBL were positive in 79.45% of the Klebsiella pneumoniae isolates. However, in the phenotypic confirmatory test for ESBL production, 53.42% of the isolates tested positive, suggesting that the phenotypic test results may be subject to false positives or negatives. Several factors can contribute to this issue, including the production of multiple β-lactamase types by a single bacterial isolate, the co-production of ESBLs and constitutive AmpC β-lactamases, variations in substrate affinities, and the inoculum effect [7]. Accordingly, in the current study, phenotypic detection revealed that only 15 isolates (34.9%) were positive for ESBL production, while genotypic detection identified 34 isolates (79%) as positive for the blaTEM gene. Therefore, PCR using oligonucleotide primers specific to ESBL genes is considered the most straightforward and reliable method for detecting the presence of ESBLs [7].
This study has several limitations that may impact the generalizability of its findings, including small sample size, sampling restricted to a specific geographic area of the city, and the exclusive focus on the blaTEM gene. Future research with larger sample sizes and broader geographic coverage, extending beyond the city to the entire country, is needed to provide deeper insights into this issue.
Conclusion
The study area shows a significant level of antibiotic resistance in KP-ESBL strains, which, if not adequately addressed, could soon lead to severe health and therapeutic consequences. Molecular methods, which detect the genes responsible for resistance, may offer greater sensitivity in identifying antibiotic resistance.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The ethics board of the College of Health Sciences at Hewler Medical University approved the study.
Patient consent (participation and publication): Written informed consent was obtained from patients for publication.
Source of Funding: Medical Research Center of Hawler Medical University.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: MAG and KAO were significant contributors to the conception of the study and the literature search for related studies. LJM, LKHJ, and KAO were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. HOA and KWM were involved in the literature review, study design, and manuscript writing. SYI and BMI Literature review, final approval of the manuscript, and processing of the tables. HOA and MAG confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.

Blood Cross Matching Without Anti-Human Globulin (AHG) and Bovine Serum: A New Interest for an Old Idea
Suhaib H. Kakamad, Mohieddin Barzegar, Mohammad Reza Rahmani
Abstract
Introduction
Transfusion medicine promotes the safety of blood transfusions by rigorously testing to eliminate risks of infection and hemolytic. The efficacy (to correct and identify antibodies) of a modified cross-matching method that excludes the use of anti-human globulin (AHG) bovine serum with that of the immediate spin crossmatch was assessed.
Material and Methods
This multi-center study was performed at two medical centers in Iran and Iraq. Over seven years, consecutive participants received two different blood cross-matching methods: one with AHG and bovine serum and another without it.
Results
The study included 31240 participants. About 18526 (59.3%) were males and 12714 (40.7%) were females. The ages of participants ranged from 23 to 57 years, with the average age of 39 years. Only 45 (0.14%) participants blood cross-match incompatible in both spin cross-match and cross-match without AHG, bovine serum the same result. The study found that all detected antibodies correlated with potential blood incompatibility, and there remained 100% safety between the two testing methods.
Conclusion
Omitting AHG and bovine serum in cross-matching might be safe. At least it can be used in emergency situations and in resource-limited settings.
Introduction
A transfusion medic is in charge of making sure the blood you are being transfused is being transfused as safely as possible. To address concerns such as transfusion-transmitted infections (TTIs) and transfusion-associated hemolytics [1], an exhaustive testing procedure is performed between the donor and recipient blood. The present red cell serology based on safe transfusion was developed in 1901 with Karl Landsteiner's identification of the ABO blood types. Later, in that same year, 1940, the Rh antigen and in the year 1946, the Kell antigen were shown to be significant red cell antigens [2]. Provided donor and recipient blood types are compatible, these minimally increase the risks of medical procedure complications, adverse reactions, and other conditions that benefit from medical procedures such as organ transplants [3,4]. Cross-matching aims to see if the blood types from the donor and receiver are compatible [5]. This is done to avoid potentially fatal immunological responses to transfused blood. This approach can search the recipient's blood for antibodies that are incompatible with the red blood cell antigens in the donor’s cells [6,7]. IgM or IgG are usually blood group antibodies, but occasionally IgA. In most cases, immune antibodies (generally IgG) and naturally occurring antibodies (predominantly IgM) [8]. The Anti human Globulin (AHG) and bovine serum detect weak antibodies, such as most IgG and some IgM antibodies. However, the bovine serum also detects weak antibodies such as IgG due to increasing its dielectric constant and hence decreasing the Zeta potential, which causes the red cell to come closer, thereby decreasing the thickness of the ionic cloud surrounding each cell, resulting in agglutination [9]. AHG helps reveal the presence of antibodies that may have coated the surface of red blood cells even when agglutination is not apparent during the initial testing phases; adding AHG and bovine to the test significantly increases the test's sensitivity [10]. These antibodies may not cause visible clumping or agglutination on their own; however, not all antigens lead to the formation of clinically significant antibodies to identify the 25–28 blood group antigens that are known to elicit hemolytic reactions (HTRs), screening for significant antibodies in the blood cells is recommended [11]. Antibody screening tests are of significant use in both the diagnosis of infectious diseases and the testing of immune systems, as well as the discovery of autoimmune conditions that can be gained from the use of antibody screening tests. As part of this procedure, the majority of blood banks routinely perform a battery of tests to identify the ABO and Rh types of both the donor and the recipient and then carry out a full cross-match of the recipient’s serum with the donor’s red blood cells to ensure transfusion stability, at times being an integral part of this procedure is a blood cross-match with bovine serum plus AHG.
The objective of the study was to assess the possibility of making the blood cross machining method safe and accurate, when AHG and bovine serum were not utilized in the process.
Material and Methods
This multi-center cohort study was conducted at the Blood Bank Department of General Hospital in Kurdistan, Iraq, and Kawsar Hospital in Sanandaj, Iran. The research spanned seven years (from December 2017 to November 2024). All patients necessitated blood transfusions, and some volunteers participated in the spin cross-match (blood cross-match with AHG, bovine) [13]. All tests were repeated blood cross-matches without AHG, bovine (Figure 1). (Inclusion criteria: patients and volunteers must possess identical ABO and Rh blood types.
Only 45 participants (1.4%) exhibited blood cross-match incompatibility in both spin cross-match and cross-match without AHG and bovine; the same result was observed where they tested positive for antibodies. Antibody screening for incompatible patients was conducted using the Bio-Rad ZE5 Cell Analyzer, a high-performance flow cytometry platform designed for a diverse array of cellular assays, providing advanced capabilities for researchers in immunology and oncology. It combines flexibility with high throughput. Data analysis each variable underwent a descriptive analysis, calculating percentages, frequencies, means, and ranges. The data were gathered on an Excel sheet. Whenever necessary, the P-value significance was set to less than 0.05.
Results
The study included 31240 participants. About 18526 (59.3%) were males and 12714 (40.7%) were females. Participants’ ages ranged from 23 to 57 years, with an average age of 39. Only 45 (1.4%) participants' blood cross was incompatible in spin cross-match and cross-match without AHG; bovine serum. From this number, 27 (53.3%) had a history of blood transfusion, 16 (59% male), and 11 (41% female). About 18 (46.7%) of women who have been pregnant at least once are included [table 1].
The results of the two tests about compatibility were identical in every way [Figure 2 and 3]. No discrepancy was found between the two procedures.
|
Alloantibodies |
Frequency (%) |
|
Anti-D |
16 (35.5%) |
|
Anti-M |
11 (24.4%) |
|
Anti-C |
9 (20%) |
|
Anti-E |
2 (4.4%) |
|
Anti-Jkb |
2 (4.4%) |
|
Anti-S |
1 (2.2%) |
|
Anti-Lea |
1 (2.2%) |
|
Anti-K |
1 (2.2%) |
|
Anti-Cw |
1 (2.2%) |
|
Anti-N |
1 (2.2%) |
|
Total |
45 (100%) |
Discussion
The International Society of Blood Transfusion has recognized 33 blood group systems, and that's a significant step forward. Other than the ABO and Rhesus systems, red blood cell membranes contain a plethora of different antigens. This is important to avoid transfusion-related problems [14]. One of the common hazards associated with red blood cell transfusions is the formation of RBC alloantibodies. Red blood cell alloimmunisation rates are notable in 1% to 35% of some groups of patients [15,16]. In this study, the alloimmunisation rate was far lower at 0.14%. The distribution of alloantibodies was determined in 35.5% (16 out of 45) of the cases where anti-D was found. About 24.4% (11 out of 45) were anti-M. The third most common group was Anti-C antibodies, at about 20%.
In Western nations, studies have shown a much higher incidence of Kell antibodies [16], which contradicts our results. This might need further studies with a broader population. The striking finding of this study was that there was not a single case where the spin cross was compatible, but without AHG, the bovine cross-match was incompatible, which is an important finding. The screening cells found every clinically relevant antibody. After the cross-match method, it was found that this screening panel is suitable for use in Iranian and Iraqi blood banks, at least in special situations like emergency settings or resource-limited regions.
This demonstrates that if the AHG bovine had been deleted from the blood and the blood was supplied using this approach, an identical result would have been observed using the AHG bovine in vitro. Identifying all clinically important antibodies is a crucial part of the pre-transfusion testing process and helps avoid an adverse response. The findings of the test are interpreted according to the presence or lack of agglutination or other markers of antigen binding [17].
On the other hand, eliminating the traditional AHG, bovine serum cross-match, may be advantageous. There is a reduction in the amount of labor that has to be done, a reduction in the prices of the reagents, a straightforward and speedy method, suitableness for emergencies, sensitivity and accuracy, fast detection of incompatible conditions, and more efficient utilization of blood inventory.
The technical staff will not have to carry out an AHG, bovine serum cross-match every ten to fifteen minutes. As a result, they will have more time to dedicate to other regions, such as donor recruiting and well-being. This cross-match without AHG bovine serum is also excellent for the development of a blood bank. A procedure for computer cross-match might potentially be created with the confidence obtained from this work in the future, provided that the software and checking points that are used are confirmed. However, confirming these benefits needs several better-designed studies with more variables.
This study has several limitations: First, although the sample size is reasonable, there is no significant data regarding the cost of the procedures in both situations. Second. The duration of both tests has not been reported. Third, we failed to report the number of agglutinations found on each test plate.
Conclusion
Based on this study's findings, crossmatching without the use of AHG and bovine serum demonstrated a predicted safety rate of 100%, yielding results comparable to those of the spin crossmatch method. This suggests that omitting AHG and bovine serum in crossmatching may be a safe alternative, particularly in emergencies and resource-limited settings, where simplifying procedures can help optimize care without compromising safety.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The ethics board of the Kurdistan University of Medical Science approved the study.
Patient consent (participation and publication): Written informed consent was obtained from patients for publication.
Source of Funding: Kurdistan University of Medical Science.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: MRR significantly contributed to the study's conception and literature search for related studies. SHK and MB were involved in the literature review, the study's design, and the critical revision of the manuscript. They also participated in data collection, the literature review, study design, and manuscript writing. MRR and SHK confirmed the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.
Review Articles

Breast Carcinoma within Fibroadenoma: A Systematic Review
Abdulwahid M. Salih, Lana R.A. Pshtiwan, Mohammed Gh. Hamasaeed, Sami S. Omar, Shaban Latif,...
Abstract
Introduction
Fibroadenoma is the most common benign breast lesion; however, it carries a potential risk of malignant transformation. This systematic review provides an overview of the presentation, management, and outcome of carcinomas arising within fibroadenomas.
Methods
A systematic search on Google Scholar was conducted for English-language studies on breast carcinoma within fibroadenomas. Studies on fibroadenomas with no malignant components, review articles, pre-prints, incomplete data, and those published in suspicious journals were excluded.
Results
On ultrasonography, 28 masses (36.8%) appeared benign, and 20 (26.3%) were suspicious, with ultrasonographic data unavailable for the remaining tumors (36.8%). Mammography data were available for 50 tumors, revealing 27 benign lesions (54%) and 23 suspicious lesions (46%). Among the 17 lesions with available magnetic resonance imaging data, five were benign lesions (29.4%), and 12 were suspicious (70.6%). Cytology evaluation among 46 tumors revealed that 20 (43.5%) were benign, 24 (52.2%) were malignant, and two (4.3%) were suspicious. The most commonly performed surgery was wide local excision (50.7%), followed by mastectomy (32.9%). On histopathology, 11 tumors exhibited more than one pathology. Ductal carcinoma in situ was the most frequent finding (40.8%), followed by invasive ductal carcinoma (28.9%) and lobular carcinoma in situ (28.4%). Recurrence was observed in one case (1.4%), and metastasis occurred in two cases (2.8%).
Conclusion
Although rare, carcinomas arising within fibroadenomas may present considerable challenges in preoperative diagnosis, whether through imaging or cytology. Therefore, clinicians may find it necessary to approach fibroadenomas with increased caution.
Introduction
Fibroadenoma is the most common benign breast lesion comprising epithelial and stromal components [1,2]. The tumor generally manifests as a hyperplastic breast lobule, presenting as a solitary mass during a woman’s early reproductive years, with the peak incidence occurring in the second and third decades of life [3,4]. Estrogen, progesterone, pregnancy, and lactation are believed to stimulate tumor growth, although it tends to shrink during menopause as estrogen levels decline [3]. Incidence rates range from 7% to 13% in the general population, with up to 20% of cases presenting with bilateral or multiple masses [3]. Clinically, fibroadenoma presents as a palpable, mobile, solid mass with a rubbery consistency and smooth, well-defined borders [5]. It is radiologically and histologically classified into simple and complex types [2]. The tumor may exceed 3 mm in size, be associated with sclerosing adenosis or epithelial calcifications, and potentially give rise to carcinomas that can invade the surrounding breast tissue. Although cases of fibroadenomas containing malignancies are rare, malignancy tends to occur more frequently in patients 10 to 20 years older than the typical age for simple fibroadenomas [2,6]. Carcinomas within fibroadenomas are most commonly carcinoma in situ (CIS) [7,8]. Invasive carcinomas, though less common, can occur, with invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) being the primary forms [6]. Carcinomas in situ signal an increased risk of developing invasive cancer if left untreated, and neoplasms arising within fibroadenomas behave similarly to those occurring independently [9]. This systematic review provides an overview of the presentation, management, and outcome of carcinomas arising within fibroadenomas.
Methods
Study design
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and search strategy
A systematic search on Google Scholar was undertaken to identify relevant English-language studies on breast carcinoma within fibroadenoma. The search strategy employed a combination of keywords, including "fibroadenoma" with terms such as (carcinoma, cancer, malignancy, malignant, carcinoma in situ, lobular carcinoma in situ (LCIS), and ductal carcinoma in situ (DCIS).
Eligibility criteria
The inclusion criteria were limited to studies specifically addressing breast carcinoma within fibroadenoma. Studies on fibroadenomas with no malignant components, review articles, pre-prints, incomplete data, and those published in suspicious journals were excluded [10].
Study selection and data extraction
Two authors independently reviewed the titles and abstracts of the identified publications. Following this, the same two authors assessed the full texts of the remaining studies based on predefined inclusion and exclusion criteria. The extracted data included the first author’s name, the country of publication, study design, patient demographics, clinical presentation, physical examination findings, imaging and cytology findings, treatment strategies, and disease prognosis.
Data analysis
Microsoft Excel (2019) was employed to collect and organize the extracted data, while data analysis (descriptive statistics) was performed using the Statistical Package for Social Sciences (SPSS), version 27.0. The results are presented as frequencies, percentages, ranges, mean with standard deviation, and medians with quartile ranges.
Results
Study selection and characteristics
A total of 317 studies were identified from the search. Thirty-six studies were excluded due to duplication (n=5) and non-English language publications (n=31). This left 281 studies for title and abstract screening. At this stage, 202 studies were excluded due to irrelevancy. As a result, 79 studies advanced to the full-text screening stage. At this point, nine studies were excluded for being meta-analyses (n=2), reviews (n=2), publications with incomplete data (n=1), and pre-prints (n=4). Nine of the remaining 70 studies were excluded for failing to meet eligibility criteria as they were published in suspicious journals [10]. Ultimately, 61 studies [1-9,11-62], encompassing 72 cases, were included (Figure 1). Most of the studies were case reports (n=58), accompanied by three case series. Most were affiliated with Japan (19.7%) and the USA (14.7%) (Table 1). The raw data of the study has been presented in Tables 1-6.
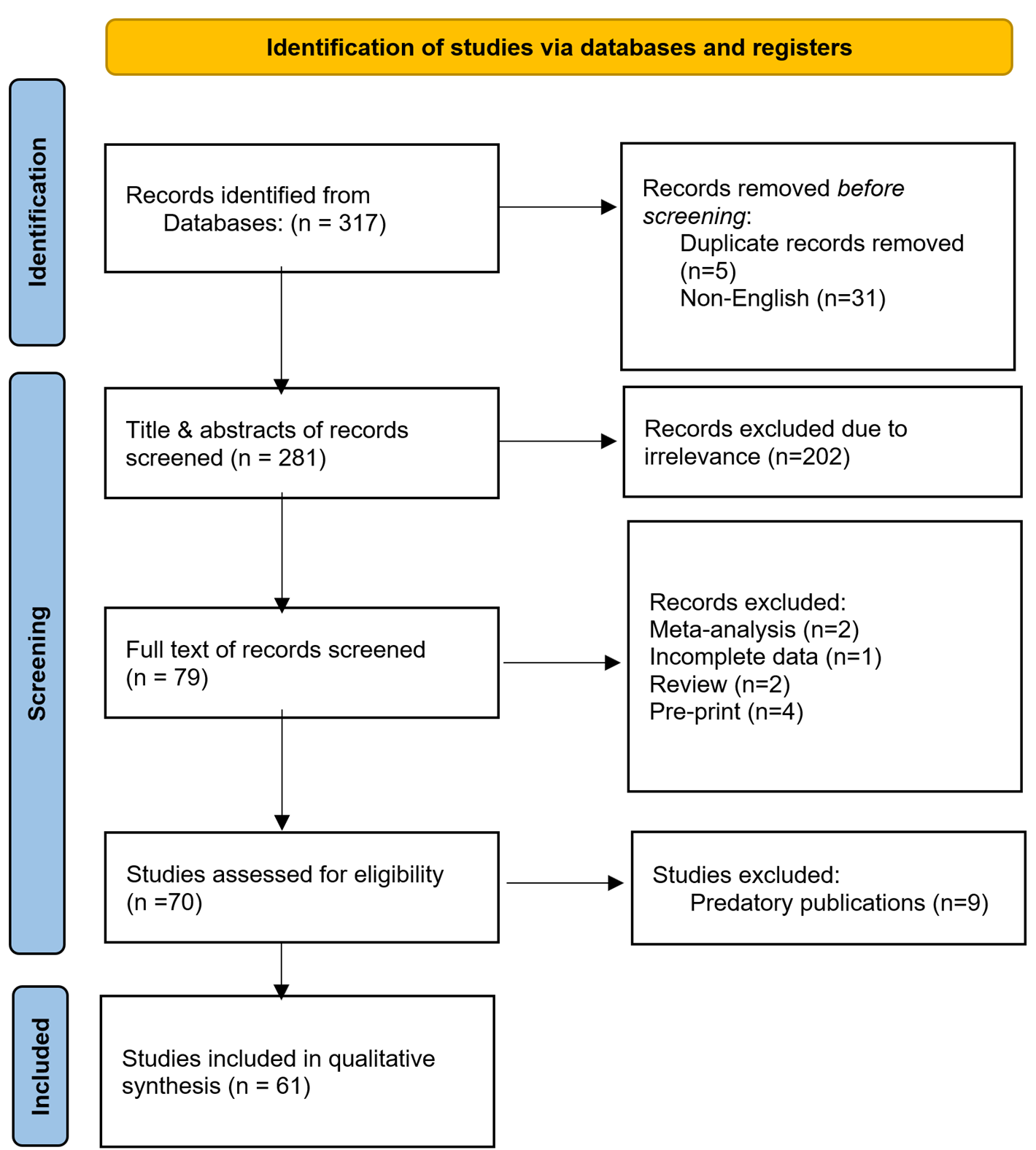
| Author /Year [reference] | Study design | No. of included case(s) | Country |
|
Ni et al./2023 [14] |
Case report |
1 |
China |
|
Brunetti et al./2023 [4] |
Case report |
1 |
Italy |
|
Wang et al./2022 [5] |
Case report |
1 |
Singapore |
|
Pang et al./2022 [2] |
Case report |
1 |
Malaysia |
|
Hammood et al./2022 [3] |
Case report |
1 |
Iraq |
|
Tagliati et al./2021 [1] |
Case report |
1 |
Italy |
|
Shojaku et al./2021 [6] |
Case report |
1 |
Japan |
|
Fujimoto et al./2021 [11] |
Case report |
1 |
Japan |
|
Feijó et al./2021[8] |
Case report |
1 |
Brazil |
|
Shiino et al./2020 [12] |
Case report |
1 |
Japan |
|
Moreno et al./2020 [17] |
Case report |
1 |
Brazil |
|
Gonthong et al./2020 [13] |
Case report |
1 |
Thailand |
|
El-Essawy et al./2020 [18] |
Case report |
1 |
KSA |
|
Brock et al./2020 [9] |
Case report |
1 |
USA |
|
Marumoto et al./2019 [16] |
Case report |
1 |
USA |
|
Zeeshan et al./2018 [19] |
Case report |
1 |
Pakistan |
|
Tiwari et al./2018 [15] |
Case report |
1 |
India |
|
Frisch et al./2018 [7] |
Case report |
1 |
South Africa |
|
Lim et al./2017 [20] |
Case report |
1 |
Korea |
|
You et al./2016 [21] |
Case report |
1 |
Korea |
|
Zheng et al./2015 [22] |
Case report |
1 |
China |
|
Hua et al./2015 [23] |
Case report |
1 |
China |
|
Wu et al./2014 [24] |
Case series |
6 |
Taiwan |
|
Mele et al./2014 [25] |
Case report |
1 |
Denmark |
|
Limite et al./2014 [26] |
Case report |
1 |
Italy |
|
Kwon et al./2014 [27] |
Case report |
1 |
Korea |
|
Kılıç et al./2014 [28] |
Case report |
1 |
Turkey |
|
Dandin et al./2014 [29] |
Case report |
1 |
Turkey |
|
Buteau et al./2014 [30] |
Case report |
1 |
USA |
|
Hayes et al./2013 [31] |
Case report |
1 |
Ireland |
|
Jahan et al./2012 [32] |
Case report |
1 |
Bangladesh |
|
Butler et al./2012 [33] |
Case report |
1 |
USA |
|
Ooe et al./2011 [34] |
Case report |
1 |
Japan |
|
Lin et al./2011 [35] |
Case report |
1 |
Taiwan |
|
Kato et al./2011 [36] |
Case report |
1 |
Japan |
|
Abu-Rahmeh et al./ 2012 [37] |
Case report |
1 |
Israel |
|
Rao et al./ 2010 [38] |
Case report |
1 |
India |
|
Petersson et al./2010 [39] |
Case report |
1 |
Singapore |
|
Tajima et al./2009 [40] |
Case report |
1 |
Japan |
|
Gashi-Luci et al./2009 [41] |
Case report |
1 |
Kosova |
|
Borecky et al./2008 [42] |
Case series |
3 |
Australia |
|
Tiu et al./2006 [43] |
Case report |
1 |
Taiwan |
|
Shin et al./2006 [44] |
Case report |
1 |
Korea |
|
Blanco et al./2005 [45] |
Case report |
1 |
USA |
|
Abite et al./2005 [46] |
Case report |
1 |
Nigeria |
|
Stafyla et al./2004 [47] |
Case report |
1 |
Greece |
|
Abe et al./ 2004 [48] |
Case report |
1 |
Japan |
|
Adelekan et al./2003 [49] |
Case report |
1 |
UK |
|
Yano et al./2001 [50] |
Case report |
1 |
Japan |
|
Gebrim et al./2000 [51] |
Case report |
1 |
Brazil |
|
Psarianos et al./1998 [52] |
Case report |
1 |
Australia |
|
Shah et al./ 1998 [53] |
Case report |
1 |
USA |
|
Kurosum et al./1994 [54] |
Case report |
1 |
Japan |
|
Morimoto et al./1993 [55] |
Case report |
1 |
Japan |
|
Gupta et al./1992 [56] |
Case report |
1 |
New Zealand |
|
Gupta et al./1991 [57] |
Case report |
1 |
New Zealand |
|
Fukud et al./1989 [58] |
Case report |
1 |
Japan |
|
Yoshida et al./1985 [59] |
Case report |
1 |
Japan |
|
Fond et al./1979 [60] |
Case report |
1 |
USA |
|
Konakry et a./1975 [61] |
Case series |
5 |
USA |
|
Durso et al./1972 [62] |
Case report |
1 |
USA |
|
First Author /Year |
Age (years) |
Gender |
Presentation |
Laterality |
Duration (months) |
PMH |
FHx of breast cancer |
|
Ni et al./2023 [14] |
60 |
F |
Mass |
UL |
12 |
NN |
Neg. |
|
Brunetti et al./2023 [4] |
35 |
F |
Lump |
UL |
NA |
NN |
FDR |
|
Wang et al./2022 [5] |
26 |
F |
Lump |
UL |
72 |
NN |
NA |
|
Pang et al./2022 [2] |
43 |
F |
Nipple discharge |
UL |
NA |
BM |
Neg. |
|
Hammood et al./2022 [3] |
49 |
F |
Lump |
UL |
60 |
BM |
Neg. |
|
Tagliati et al./2021 [1] |
49 |
F |
Lump |
UL |
NA |
NA |
Neg. |
|
Shojaku et al./2021 [6] |
61 |
F |
Mass |
UL |
60 |
NN |
Neg. |
|
Fujimoto et al./2021 [11] |
31 |
F |
Mass |
UL |
12 |
NN |
Neg. |
|
Feijó et al./2021[8] |
31 |
F |
Lump |
UL |
48 |
NA |
Neg. |
|
Shiino et al./2020 [12] |
53 |
F |
Lump |
UL |
156 |
NA |
NA |
|
Moreno et al./2020 [17] |
58 |
F |
Lump |
UL |
NA |
NA |
NA |
|
Gonthong et al./2020 [13] |
38 |
F |
Mass |
UL |
NA |
IDC |
NA |
|
El-Essawy et al./2020 [18] |
25 |
F |
Mass |
UL |
1 |
MBBM |
Neg. |
|
Brock et al./2020 [9] |
27 |
F |
Lump |
UL |
4 |
FBD |
NA |
|
Marumoto et al./2019 [16] |
70 |
F |
Mass |
UL |
NA |
NA |
Neg. |
|
Zeeshan et al./2018 [19] |
34 |
F |
Lump |
UL |
12 |
NN |
NA |
|
Tiwari et al./2018 [15] |
28 |
F |
Lump |
BL |
96 |
NN |
Neg. |
|
Frisch et al./2018 [7] |
18 |
F |
Lump |
UL |
48 |
NN |
Neg. |
|
Lim et al./2017 [20] |
20 |
F |
Nodule |
UL |
NA |
NN |
Neg. |
|
You et al./2016 [21] |
38 |
F |
Incidental |
UL |
NA |
NA |
Neg. |
|
Zheng et al./2015 [22] |
48 |
F |
Lump |
BL |
NA |
NA |
NA |
|
Hua et al./2015 [23] |
44 |
F |
Lump |
BL |
12 |
NA |
NA |
|
Wu et al./2014 [24]
|
39 |
F |
NA |
NA |
24 |
NA |
NA |
|
31 |
F |
NA |
NA |
84 |
NA |
NA |
|
|
30 |
F |
NA |
NA |
NA |
NA |
NA |
|
|
63 |
F |
NA |
NA |
0.5 |
NA |
NA |
|
|
48 |
F |
NA |
NA |
3 |
NA |
NA |
|
|
40 |
F |
NA |
NA |
0 |
NA |
NA |
|
|
Mele et al./2014 [25] |
63 |
F |
NA |
UL |
NA |
NA |
Pos. |
|
Limite et al./2014 [26] |
26 |
F |
Lump |
UL |
NA |
NA |
Neg. |
|
Kwon et al./2014 [27] |
20 |
F |
Lump |
BL |
1 |
NN |
Neg. |
|
Kılıç et al./2014 [28] |
30 |
F |
Mass |
UL |
NA |
NA |
Neg. |
|
Dandin et al./2014 [29] |
35 |
F |
Mass |
UL |
1.5 |
NN |
Neg. |
|
Buteau et al./2014 [30] |
59 |
F |
Mass |
UL |
36 |
NN |
Neg. |
|
Hayes et al./2013 [31] |
51 |
F |
Incidental |
NA |
NA |
NA |
NA |
|
Jahan et al./2012 [32] |
55 |
F |
Lump |
BL |
240 |
NA |
NA |
|
Butler et al./2012 [33] |
46 |
F |
Mass |
NA |
60 |
NA |
NA |
|
Ooe et al./2011 [34] |
46 |
F |
Lump |
UL |
60 |
NN |
Neg. |
|
Lin et al./2011 [35] |
34 |
F |
Lump |
UL |
NA |
NN |
Neg. |
|
Kato et al./2011 [36] |
42 |
F |
Mass |
UL |
NA |
NA |
NA |
|
Abu-Rahmeh et al./ 2012 [37] |
69 |
F |
Mass |
UL |
168 |
NA |
FDR |
|
Rao et al./ 2010 [38] |
30 |
F |
Lump |
UL |
1 |
NN |
Neg. |
|
Petersson et al./2010 [39] |
49 |
F |
Incidental |
UL |
48 |
NA |
NA |
|
Tajima et al./2009 [40] |
60 |
F |
Mass |
UL |
3 |
NA |
NA |
|
Gashi-Luci et al./2009 [41] |
39 |
F |
Lump |
UL |
2 |
NA |
Neg. |
|
Borecky et al./2008 [42] |
64 |
F |
Mass |
UL |
NA |
NA |
NA |
|
80 |
F |
Lump |
UL |
600 |
NA |
NA |
|
|
53 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
Tiu et al./2006 [43] |
45 |
F |
Lump |
UL |
60 |
NN |
NA |
|
Shin et al./2006 [44] |
51 |
F |
Mass |
UL |
12 |
NN |
Neg. |
|
Blanco et al./2005 [45] |
63 |
F |
Mass |
UL |
60 |
NN |
Neg. |
|
Abite et al./2005 [46] |
23 |
F |
Lump |
UL |
12 |
NA |
Neg. |
|
Stafyla et al./2004 [47] |
27 |
F |
Mass |
UL |
NA |
NA |
NA |
|
Abe et al./ 2004 [48] |
42 |
F |
Lump |
UL |
3 |
NN |
Neg. |
|
Adelekan et al./2003 [49] |
61 |
F |
Lump |
BL |
120, 0.75 |
NA |
NA |
|
Yano et al./2001 [50] |
54 |
F |
Mass |
UL |
36 |
NA |
Neg. |
|
Gebrim et al./2000 [51] |
58 |
F |
Nodule |
UL |
NA |
NA |
NA |
|
Psarianos et al./1998 [52] |
34 |
F |
Mass |
UL |
NA |
NA |
NA |
|
Shah et al./ 1998 [53] |
45 |
F |
Mass |
UL |
0.25 |
NA |
Neg. |
|
Kurosum et al./1994 [54] |
42 |
F |
Lump |
UL |
21 |
NA |
NA |
|
Morimoto et al./1993 [55] |
49 |
F |
Lump |
UL |
2 |
NA |
NA |
|
Gupta et al./1992 [56] |
59 |
F |
Mass |
UL |
0.5 |
NN |
Neg. |
|
Gupta et al./1991 [57] |
49 |
F |
Mass |
UL |
7 |
NA |
Neg. |
|
Fukud et al./1989 [58] |
45 |
F |
Lump |
UL |
NA |
BM |
NA |
|
Yoshida et al./1985 [59] |
58 |
F |
Lump |
UL |
1 |
HTN |
Neg. |
|
Fond et al./1979 [60] |
27 |
F |
Lump |
UL |
NA |
CAH |
SDR |
|
Konakry et a./1975 [61] |
59 |
F |
NA |
UL |
NA |
NA |
NA |
|
39 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
44 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
46 |
F |
NA |
UL |
NA |
DCIS |
NA |
|
|
48 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
Durso et al./1972 [62] |
42 |
F |
Lump |
UL |
NA |
NA |
NA |
| F: female, PMH: Past Medical History, FHx: Family History, UL: Unilateral, BL: bilateral, NA: Non-available, BM: Breast Mass, NN: Nothing Noteworthy, IDC: Invasive Ductal Carcinoma, MBBM: Multiple Bilateral Breast Mass, FBD: Fibrocystic Breast Disease, HTN: Hypertension, CAH: Congenital Adrenal Hyperplasia, DCIS: Ductal Carcinoma In Situ, FDR: First-Degree Relative, SDR: Second-Degree Relative, Neg.: Negative, Pos.: Positive. | |||||||
| First Author. /Year | Physical examination |
Ax LAD |
Size |
Location |
Shape |
Margin |
Vascularity |
Calcification |
||
|
Surface |
Consistency |
Mobility |
||||||||
|
Ni et al./2023 [14] |
NA |
NA |
NM |
Neg. |
7.7 mm |
RUA |
Round |
Smooth |
NA |
Pos. |
|
Brunetti et al./2023 [4] |
NA |
NA |
M |
Neg. |
15 mm |
LLA |
Oval |
Well defined |
NA |
NA |
|
Wang et al./2022 [5] |
NA |
NA |
NA |
NA |
24 mm |
LT |
NA |
Irregular |
NA |
NA |
|
Pang et al./2022 [2] |
NA |
NA |
NA |
NA |
16.7 mm |
ROA |
Oval |
Lobulated |
Moderate |
Neg. |
|
Hammood et al./2022 [3] |
Smooth |
Firm |
NM |
NA |
9.5mm |
RT |
Oval |
Well defined |
NA |
NA |
|
Tagliati et al./2021 [1] |
NA |
NA |
NA |
NA |
35 mm |
RT |
Oval |
Well defined |
NA |
NA |
|
Shojaku et al./2021 [6] |
NA |
Hard |
NA |
NA |
11.9 mm |
LT |
Oval |
Well defined |
NA |
Neg. |
|
Fujimoto et al./2021 [11] |
NA |
NA |
NA |
Neg. |
22 mm |
LT |
NA |
Well defined |
NA |
Pos. |
|
Feijó et al./2021[8] |
NA |
NA |
NA |
Neg. |
30 mm |
LUOQ |
NA |
Well defined |
Neg. |
Neg. |
|
Shiino et al./2020 [12] |
NA |
Hard |
NA |
Pos. |
36 mm |
RLIQ |
NA |
Ill defined |
NA |
Pos. |
|
Moreno et al./2020 [17] |
NA |
NA |
NA |
Pos. |
9.8 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
Gonthong et al./2020 [13] |
NA |
NA |
NA |
NA |
20 mm |
RT |
Oval |
Microlobulated |
NA |
Pos. |
|
El-Essawy et al./2020 [18] |
NA |
NA |
NA |
NA |
28.7 mm |
LIA |
NA |
Irregular |
Increased |
Pos. |
|
Brock et al./2020 [9] |
NA |
Firm |
M |
NA |
19.8 mm |
LOA |
NA |
NA |
NA |
Neg. |
|
Marumoto et al./2019 [16] |
NA |
NA |
M |
Neg. |
20.4 mm |
RUOQ |
NA |
Irregular |
NA |
Neg. |
|
Zeeshan et al./2018 [19] |
NA |
NA |
M |
NA |
47.9 mm |
RRA |
NA |
Lobulated |
NA |
NA |
|
Tiwari et al./2018 [15] |
Smooth |
Firm |
M |
NA |
NA |
BL |
NA |
Well defined |
NA |
NA |
|
Frisch et al./2018 [7] |
NA |
NA |
M |
Neg. |
39.3 mm |
RLIQ |
NA |
Well defined |
Neg. |
Neg. |
|
Lim et al./2017 [20] |
NA |
NA |
NA |
NA |
64.8 mm |
RUA |
NA |
NA |
NA |
NA |
|
You et al./2016 [21] |
NA |
NA |
NA |
Neg. |
6.9 mm |
RUIQ |
Oval |
Well defined |
NA |
Pos. |
|
Zheng et al./2015 [22] |
NA, Smooth |
NA, NA |
NA, M |
Neg., Neg. |
24.5 mm, NA |
LUA, RUIQ |
NA, NA |
Ill defined, well defined |
NA, NA |
NA, Pos. |
|
Hua et al./2015 [23] |
NA |
NA |
NA |
NA |
22.4 mm |
LT |
NA |
Well defined |
Moderate |
Pos. |
|
Wu et al./2014 [24] |
NA |
NA |
NA |
NA |
27 mm |
NA |
NA |
NA |
NA |
NA |
|
NA |
NA |
NA |
NA |
34.5 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
14.5 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
12 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
9 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
6 mm |
NA |
NA |
NA |
NA |
NA |
|
|
Mele et al./2014 [25] |
NA |
NA |
NA |
Pos. |
50 mm |
LLOQ |
NA |
Well defined |
NA |
Pos. |
|
Limite et al./2014 [26] |
Smooth |
Hard |
M |
Neg. |
1.8 mm |
RLA |
NA |
Ill defined |
NA |
NA |
|
Kwon et al./2014 [27] |
NA, NA |
Firm, Firm |
M, M |
Neg., Neg. |
16.9 mm, 21.9 mm |
RT, LT |
NA, Oval |
Lobulated, Irregular |
NA, NA |
Pos., Pos. |
|
Kılıç et al./2014 [28] |
NA |
Firm |
NA |
Neg. |
19.9 mm |
LRA |
NA |
Well defined |
NA |
Pos. |
|
Dandin et al./2014 [29] |
NA |
NA |
M |
Neg. |
11.8 mm |
LUOQ |
Oval |
Irregular |
NA |
NA |
|
Buteau et al./2014 [30] |
NA |
NA |
NA |
Pos. |
17 mm |
LT |
Lobular |
NA |
NA |
NA |
|
Hayes et al./2013 [31] |
NA |
NA |
NA |
NA |
35 mm |
NA |
Multilobulated |
Circumscribed |
NA |
Pos. |
|
Jahan et al./2012 [32] |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
39.2 mm, 36.3 mm |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
|
Butler et al./2012 [33] |
NA |
NA |
NA |
NA |
7.3 mm |
NA |
Oval |
Well defined |
NA |
NA |
|
Ooe et al./2011 [34] |
Smooth |
Firm |
M |
Neg. |
25 mm |
RUOQ |
Oval |
Well defined |
Increased |
Pos. |
|
Lin et al./2011 [35] |
NA |
NA |
M |
Neg. |
NA |
RUA |
Oval |
Well defined |
NA |
Pos. |
|
Kato et al./2011 [36] |
NA |
Hard |
NA |
NA |
15 mm |
RT |
Irregular |
NA |
NA |
Pos. |
|
Abu-Rahmeh et al./ 2012 [37] |
NA |
NA |
NA |
NA |
50 mm |
LT |
NA |
Well defined |
NA |
NA |
|
Rao et al./ 2010 [38] |
NA |
Firm |
M |
NA |
28.3 mm |
RUA |
Oval |
Smooth |
NA |
Pos. |
|
Petersson et al./2010 [39] |
NA |
NA |
NA |
NA |
30 mm |
NA |
NA |
Well defined |
NA |
NA |
|
Tajima et al./2009 [40] |
NA |
NA |
M |
NA |
16.6 mm |
RUIQ |
Lobular |
Irregular |
NA |
Pos. |
|
Gashi-Luci et al./2009 [41] |
NA |
NA |
NA |
Neg. |
20 mm |
RUOQ |
NA |
NA |
NA |
NA |
|
Borecky et al./2008 [42] |
NA |
NA |
NA |
NA |
12 mm |
LT |
NA |
Irregular |
NA |
Pos. |
|
NA |
NA |
NA |
NA |
40 mm |
LUIQ |
NA |
Ill defined |
NA |
Pos. |
|
|
NA |
NA |
NA |
NA |
17 mm |
NA |
Oval |
Well defined |
NA |
Pos. |
|
|
Tiu et al./2006 [43] |
NA |
NA |
M |
Neg. |
13 mm |
LUOQ |
NA |
Well defined |
Increased |
NA |
|
Shin et al./2006 [44] |
NA |
NA |
M |
Neg. |
12.3 mm |
RUIQ |
Oval |
Well defined |
Pos. |
Pos. |
|
Blanco et al./2005 [45] |
NA |
NA |
NA |
NA |
17.5 mm |
RT |
Round |
Well defined |
NA |
Pos. |
|
Abite et al./2005 [46] |
NA |
Firm |
M |
Neg. |
34.2 mm |
RUOQ |
NA |
Well defined |
NA |
NA |
|
Stafyla et al./2004 [47] |
NA |
NA |
M |
Neg. |
34 mm |
RUOQ |
NA |
Well defined |
NA |
NA |
|
Abe et al./ 2004 [48] |
NA |
Firm |
NA |
Neg. |
47.4 mm |
LUOQ |
Irregular |
Well defined |
NA |
Neg. |
|
Adelekan et al./2003 [49] |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
35 mm, 60 mm |
NA, LUIQ |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
|
Yano et al./2001 [50] |
Smooth |
Hard |
M |
Neg. |
18.8 mm |
LUIQ |
NA |
Well defined |
Minimal |
Neg. |
|
Gebrim et al./2000 [51] |
NA |
NA |
M |
Neg. |
24.5 mm |
LT |
NA |
Well defined |
NA |
Neg. |
|
Psarianos et al./1998 [52] |
NA |
Firm |
M |
NA |
29.7 mm |
RUIQ |
NA |
Well defined |
NA |
NA |
|
Shah et al./ 1998 [53] |
NA |
Firm |
M |
Neg. |
22.4 mm |
RUIQ |
NA |
Well defined |
NA |
NA |
|
Kurosum et al./1994 [54] |
NA |
Rubbery |
NA |
Neg. |
22.9 mm |
RUOQ |
NA |
Well defined |
NA |
NA |
|
Morimoto et al./1993 [55] |
NA |
Rubbery |
M |
NA |
24.5 mm |
LUIQ |
NA |
Well defined |
NA |
NA |
|
Gupta et al./1992 [56] |
NA |
Firm |
NA |
NA |
19.4 mm |
LT |
NA |
NA |
NA |
NA |
|
Gupta et al./1991 [57] |
NA |
Rubbery |
NA |
NA |
NA |
LUOQ |
NA |
NA |
NA |
NA |
|
Fukud et al./1989 [58] |
Smooth |
NA |
NA |
NA |
39.2 mm |
ROA |
NA |
NA |
NA |
Neg. |
|
Yoshida et al./1985 [59] |
Smooth |
Firm |
PM |
Neg. |
34.1 mm |
LUOQ |
NA |
Well defined |
High |
Neg. |
|
Fond et al./1979 [60] |
NA |
NA |
M |
NA |
20 mm |
RSA |
NA |
NA |
NA |
NA |
|
Konakry et a./1975 [61] |
NA |
NA |
NA |
NA |
20 mm |
RUOQ |
NA |
NA |
NA |
Pos. |
|
NA |
NA |
NA |
NA |
50 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
20 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
8 mm |
RUOQ |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
31.1 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
|
Durso et al./1972 [62] |
Smooth |
NA |
NA |
NA |
15 mm |
RUIQ |
NA |
NA |
NA |
NA |
| N/A: Non-available, mm: Millimeters, Ax LAD: Axillary Lymphadenopathy, RUA: Right Upper Aspect, LLA: Left Lower Aspect, LT: Left, RT: Right, ROA: Right Outer Aspect, LUIQ: Left Upper Inner Quadrant, RLOQ: Right Lower Outer Quadrant, LUOQ: Left Upper Outer Quadrant, RLIQ: Right Lower Inner Quadrant, LIA: Left Inner Aspect, LOA: Left Outer Aspect, RUOQ: Right Upper Outer Quadrant, RRA: Right Retro-Areolar, BL: Bilateral, RLA: Right Lower Aspect, LRA: Left Retro-Areolar, LLOQ: Left Lower Outer Quadrant, LUA: Left Upper Aspect, RIA: Right Inner Aspect, RUIQ: Right Upper Inner Quadrant, RSA: Right Subareolar Area, Neg.: Negative, Pos.: Positive, NM: Non-Mobile, M: Mobile, PM: Partially mobile. | ||||||||||
| First Author. /Year |
Radiological findings |
Pre-operative diagnosis (CNB or FNAC) | ||
|
U/S |
MMG |
MRI |
||
|
Ni et al./2023 [14] |
Benign |
Benign |
Suspicious |
N/A |
|
Brunetti et al./2023 [4] |
Suspicious |
Benign |
N/A |
DCIS |
|
Wang et al./2022 [5] |
Benign |
N/A |
N/A |
Benign |
|
Pang et al./2022 [2] |
Benign |
Benign |
N/A |
Benign |
|
Hammood et al./2022 [3] |
Benign |
Benign |
Benign |
Benign |
|
Tagliati et al./2021 [1] |
Benign |
N/A |
Suspicious |
Benign |
|
Shojaku et al./2021 [6] |
Benign |
Benign |
Suspicious |
Malignant |
|
Fujimoto et al./2021 [11] |
Suspicious |
Suspicious |
Benign |
IDC |
|
Feijó et al./2021[8] |
Benign |
N/A |
N/A |
Suspicious |
|
Shiino et al./2020 [12] |
Suspicious |
Suspicious |
Suspicious |
IDC |
|
Moreno et al./2020 [17] |
N/A |
N/A |
N/A |
N/A |
|
Gonthong et al./2020 [13] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
El-Essawy et al./2020 [18] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
Brock et al./2020 [9] |
Benign |
Benign |
N/A |
Benign |
|
Marumoto et al./2019 [16] |
Suspicious |
Benign |
N/A |
Benign |
|
Zeeshan et al./2018 [19] |
Suspicious |
Suspicious |
N/A |
Benign |
|
Tiwari et al./2018 [15] |
Benign |
N/A |
N/A |
Benign |
|
Frisch et al./2018 [7] |
Benign |
N/A |
N/A |
N/A |
|
Lim et al./2017 [20] |
N/A |
N/A |
N/A |
N/A |
|
You et al./2016 [21] |
Suspicious |
Suspicious |
N/A |
Suspicious |
|
Zheng et al./2015 [22] |
Suspicious, Benign |
N/A, N/A |
N/A, N/A |
N/A, N/A |
|
Hua et al./2015 [23] |
Suspicious |
Suspicious |
N/A |
Benign |
|
Wu et al./2014 [24]
|
N/A |
N/A |
N/A |
N/A |
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
Mele et al./2014 [25] |
Benign |
Suspicious |
Suspicious |
IAC |
|
Limite et al./2014 [26] |
Benign |
N/A |
N/A |
N/A |
|
Kwon et al./2014 [27] |
Benign, Benign |
N/A, N/A |
N/A, N/A |
Benign, Benign |
|
Kılıç et al./2014 [28] |
Benign |
Suspicious |
Benign |
DCIS |
|
Dandin et al./2014 [29] |
Suspicious |
N/A |
N/A |
N/A |
|
Buteau et al./2014 [30] |
N/A |
Benign |
Benign |
Benign |
|
Hayes et al./2013 [31] |
N/A |
Suspicious |
N/A |
Benign |
|
Jahan et al./2012 [32] |
Benign, Benign |
N/A, N/A |
N/A, N/A |
N/A, N/A |
|
Butler et al./2012 [33] |
Benign |
Benign |
N/A |
ILC – LCIS |
|
Ooe et al./2011 [34] |
Suspicious |
Benign |
Suspicious |
DCIS |
|
Lin et al./2011 [35] |
Benign |
Suspicious |
N/A |
IDC - DCIS |
|
Kato et al./2011 [36] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
Abu-Rahmeh et al./ 2012 [37] |
Benign |
Benign |
N/A |
IDC |
|
Rao et al./ 2010 [38] |
Benign |
Benign |
N/A |
Malignant |
|
Petersson et al./2010 [39] |
N/A |
Benign |
N/A |
N/A |
|
Tajima et al./2009 [40] |
Suspicious |
Suspicious |
Suspicious |
Malignant |
|
Gashi-Luci et al./2009 [41] |
Suspicious |
Suspicious |
N/A |
Benign |
|
Borecky et al./2008 [42] |
Suspicious |
Suspicious |
N/A |
IDC - DCIS |
|
Suspicious |
Suspicious |
N/A |
IC |
|
|
Suspicious |
Suspicious |
N/A |
IDC - DCIS |
|
|
Tiu et al./2006 [43] |
Benign |
Benign |
N/A |
Malignant |
|
Shin et al./2006 [44] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
Blanco et al./2005 [45] |
N/A |
Benign |
N/A |
N/A |
|
Abite et al./2005 [46] |
N/A |
N/A |
N/A |
N/A |
|
Stafyla et al./2004 [47] |
Benign |
N/A |
N/A |
N/A |
|
Abe et al./ 2004 [48] |
Suspicious |
Benign |
N/A |
Benign |
|
Adelekan et al./2003 [49] |
N/A, N/A |
Benign, Benign |
N/A, N/A |
Benign, Malignant |
|
Yano et al./2001 [50] |
Benign |
Suspicious |
Benign |
Malignant |
|
Gebrim et al./2000 [51] |
N/A |
Suspicious |
N/A |
Benign |
|
Psarianos et al./1998 [52] |
Benign |
Benign |
N/A |
N/A |
|
Shah et al./ 1998 [53] |
N/A |
Benign |
N/A |
Benign |
|
Kurosum et al./1994 [54] |
Benign |
N/A |
N/A |
N/A |
|
Morimoto et al./1993 [55] |
N/A |
N/A |
N/A |
Benign |
|
Gupta et al./1992 [56] |
N/A |
Suspicious |
N/A |
Malignant |
|
Gupta et al./1991 [57] |
N/A |
Benign |
N/A |
Malignant |
|
Fukud et al./1989 [58] |
Benign |
Benign |
N/A |
N/A |
|
Yoshida et al./1985 [59] |
N/A |
Suspicious |
Suspicious |
N/A |
|
Fond et al./1979 [60] |
N/A |
N/A |
N/A |
Benign |
|
Konakry et a./1975 [61] |
N/A |
Suspicious |
N/A |
N/A |
|
N/A |
Benign |
N/A |
N/A |
|
|
N/A |
Benign |
N/A |
N/A |
|
|
N/A |
Benign |
N/A |
N/A |
|
|
N/A |
Benign |
N/A |
N/A |
|
|
Durso et al./1972 [62] |
N/A |
Benign |
N/A |
N/A |
| N/A: non-available, U/S: Ultrasound, MMG: Mammogram, MRI: Magnetic Resonance Imaging, CNB: Core Needle Biopsy, FNAC: Fine Needle Aspiration Cytology, DCIS: Ductal Carcinoma In Situ, IDC: Invasive Ductal Carcinoma, CIS: Carcinoma In Situ, IAC: Invasive apocrine carcinoma, ILC: Invasive Lobular Carcinoma, LCIS: Lobular Carcinoma In Suspicious, IC: Invasive Carcinoma. | ||||
|
First Author /Year |
Management |
Hormonal therapy |
|||
|
Breast surgery |
Axillary surgery |
Chemotherapy |
Radiotherapy | ||
|
Ni et al./2023 [14] |
WLE |
SLNB |
No |
No |
NA |
|
Brunetti et al./2023 [4] |
WLE |
ALND |
Yes |
NA |
NA |
|
Wang et al./2022 [5] |
EB |
None |
NA |
Yes |
Yes |
|
Pang et al./2022 [2] |
WLE |
None |
No |
NA |
Yes |
|
Hammood et al./2022 [3] |
WLE |
None |
NA |
NA |
Yes |
|
Tagliati et al./2021 [1] |
WLE |
None |
NA |
No |
NA |
|
Shojaku et al./2021 [6] |
WLE |
SLNB |
NA |
Yes |
NA |
|
Fujimoto et al./2021 [11] |
WLE |
SLNB |
Yes |
Yes |
NA |
|
Feijó et al./2021[8] |
WLE |
None |
Yes |
Yes |
NA |
|
Shiino et al./2020 [12] |
MX |
ALND |
Yes |
Yes |
NA |
|
Moreno et al./2020 [17] |
MX |
None |
NA |
NA |
NA |
|
Gonthong et al./2020 [13] |
MX |
ALND |
No |
No |
Yes |
|
El-Essawy et al./2020 [18] |
WLE |
None |
NA |
NA |
NA |
|
Brock et al./2020 [9] |
WLE |
None |
NA |
NA |
NA |
|
Marumoto et al./2019 [16] |
EB |
None |
No |
Yes |
Yes |
|
Zeeshan et al./2018 [19] |
WLE |
None |
NA |
Yes |
Yes |
|
Tiwari et al./2018 [15] |
WLE |
None |
No |
No |
NA |
|
Frisch et al./2018 [7] |
WLE |
NA |
NA |
No |
Yes |
|
Lim et al./2017 [20] |
WLE |
None |
No |
No |
No |
|
You et al./2016 [21] |
WLE |
None |
NA |
NA |
Yes |
|
Zheng et al./2015 [22] |
MX |
ALND |
Yes |
NA |
Yes |
|
Hua et al./2015 [23] |
MX |
None |
NA |
NA |
Yes |
|
Wu et al./2014 [24]
|
WLE |
SLNB |
No |
No |
Yes |
|
MX |
ALND |
Yes |
No |
Yes |
|
|
WLE |
NA |
No |
No |
Yes |
|
|
WLE |
SLNB |
No |
Yes |
Yes |
|
|
WLE |
SLNB |
No |
No |
No |
|
|
MX |
SLNB |
No |
No |
Yes |
|
|
Mele et al./2014 [25] |
MRM |
ALND |
NA |
NA |
NA |
|
Limite et al./2014 [26] |
WLE |
SLNB |
No |
No |
NA |
|
Kwon et al./2014 [27] |
WLE |
None |
NA |
Yes |
NA |
|
Kılıç et al./2014 [28] |
WLE |
None |
NA |
NA |
NA |
|
Dandin et al./2014 [29] |
WLE |
ALND |
Yes |
NA |
NA |
|
Buteau et al./2014 [30] |
WLE |
ALND |
Yes |
Yes |
Yes |
|
Hayes et al./2013 [31] |
WLE |
SLNB |
NA |
NA |
NA |
|
Jahan et al./2012 [32] |
WLE |
None |
NA |
NA |
NA |
|
Butler et al./2012 [33] |
WLE |
None |
NA |
NA |
NA |
|
Ooe et al./2011 [34] |
WLE |
SLNB |
No |
Yes |
Yes |
|
Lin et al./2011 [35] |
MRM |
None |
NA |
NA |
NA |
|
Kato et al./2011 [36] |
WLE |
SLNB |
NA |
NA |
NA |
|
Abu-Rahmeh et al./ 2012 [37] |
NA |
NA |
NA |
NA |
NA |
|
Rao et al./ 2010 [38] |
MRM |
ALND |
NA |
NA |
NA |
|
Petersson et al./2010 [39] |
EB |
SLNB |
NA |
NA |
NA |
|
Tajima et al./2009 [40] |
WLE |
None |
NA |
NA |
NA |
|
Gashi-Luci et al./2009 [41] |
RM |
ALND |
NA |
NA |
NA |
|
Borecky et al./2008 [42] |
NA |
NA |
NA |
NA |
NA |
|
NA |
NA |
NA |
NA |
NA |
|
|
EB |
SLNB |
NA |
NA |
NA |
|
|
Tiu et al./2006 [43] |
MX |
None |
NA |
NA |
NA |
|
Shin et al./2006 [44] |
MX |
SLNB |
NA |
NA |
NA |
|
Blanco et al./2005 [45] |
WLE |
SLNB |
NA |
NA |
NA |
|
Abite et al./2005 [46] |
EB |
None |
NA |
NA |
NA |
|
Stafyla et al./2004 [47] |
EB |
None |
No |
No |
NA |
|
Abe et al./ 2004 [48] |
MX |
ALND |
Yes |
NA |
Yes |
|
Adelekan et al./2003 [49] |
EB, MRM |
None, ALND |
Yes |
Yes |
Yes |
|
Yano et al./2001 [50] |
WLE |
ALND |
NA |
Yes |
NA |
|
Gebrim et al./2000 [51] |
MX |
ALND |
NA |
NA |
NA |
|
Psarianos et al./1998 [52] |
EB |
None |
NA |
NA |
NA |
|
Shah et al./ 1998 [53] |
WLE |
NA |
NA |
NA |
NA |
|
Kurosum et al./1994 [54] |
WLE |
None |
NA |
Yes |
NA |
|
Morimoto et al./1993 [55] |
WLE |
None |
Yes |
NA |
NA |
|
Gupta et al./1992 [56] |
WLE |
None |
NA |
Yes |
Yes |
|
Gupta et al./1991 [57] |
WLE |
ALND |
NA |
Yes |
NA |
|
Fukud et al./1989 [58] |
MRM |
NA |
NA |
NA |
NA |
|
Yoshida et al./1985 [59] |
RM |
ALND |
No |
No |
NA |
|
Fond et al./1979 [60] |
MRM |
ALND |
NA |
NA |
NA |
|
Konakry et a./1975 [61] |
RM |
NA |
NA |
NA |
NA |
|
MRM |
NA |
NA |
NA |
NA |
|
|
MRM |
NA |
NA |
NA |
NA |
|
|
MX |
NA |
NA |
NA |
NA |
|
|
MRM |
NA |
NA |
NA |
NA |
|
|
Durso et al./1972 [62] |
EB |
None |
NA |
NA |
NA |
| NA: non-available, WLE: Wide Local Excision, EB: Excisional Biopsy, RM: Radical Mastectomy, MRM: Modified Radical Mastectomy, MX: Mastectomy, ALND: Axillary Lymph Node Dissection, SLNB: Sentinel Lymph Node Biopsy. | |||||
|
First Author /Year |
Post-operative HPE |
Immunohistochemistry (ER-PR-HER2) |
Axillary status |
FU (months) |
Recurrence |
Metastasis |
|
Ni et al./2023 [14] |
DCIS |
ER - PR |
Neg. |
NA |
NA |
No |
|
Brunetti et al./2023 [4] |
IDC |
TN |
Pos. |
NA |
NA |
Yes |
|
Wang et al./2022 [5] |
ILC - LCIS |
ER – PR |
NA |
NA |
NA |
NA |
|
Pang et al./2022 [2] |
LCIS |
NA |
NA |
4 |
No |
No |
|
Hammood et al./2022 [3] |
DCIS |
NA |
NA |
NA |
No |
No |
|
Tagliati et al./2021 [1] |
DCIS |
ER – PR |
NA |
60 |
No |
No |
|
Shojaku et al./2021 [6] |
DCIS |
ER |
NA |
24 |
No |
No |
|
Fujimoto et al./2021 [11] |
IDC |
HER2 |
Neg. |
6 |
No |
No |
|
Feijó et al./2021[8] |
DCIS |
ER – PR |
NA |
48 |
No |
No |
|
Shiino et al./2020 [12] |
IDC |
TN |
Neg. |
30 |
No |
No |
|
Moreno et al./2020 [17] |
LCIS |
NA |
NA |
120 |
No |
No |
|
Gonthong et al./2020 [13] |
DCIS |
TN |
Neg. |
12 |
No |
No |
|
El-Essawy et al./2020 [18] |
NA |
TN |
NA |
NA |
NA |
NA |
|
Brock et al./2020 [9] |
LCIS |
NA |
NA |
NA |
NA |
NA |
|
Marumoto et al./2019 [16] |
DCIS |
ER |
NA |
12 |
No |
No |
|
Zeeshan et al./2018 [19] |
DCIS |
ER – PR |
NA |
NA |
NA |
No |
|
Tiwari et al./2018 [15] |
DCIS |
NA |
NA |
12 |
No |
No |
|
Frisch et al./2018 [7] |
DCIS |
ER |
NA |
NA |
NA |
No |
|
Lim et al./2017 [20] |
CA |
TN |
NA |
21 |
No |
No |
|
You et al./2016 [21] |
DCIS |
ER – PR |
NA |
52 |
No |
No |
|
Zheng et al./2015 [22] |
ILC, IDC |
HER2, ER-PR-HER2 |
Neg., Neg. |
3 |
No |
No |
|
Hua et al./2015 [23] |
LCIS |
ER – PR |
NA |
60 |
No |
No |
|
Wu et al./2014 [24]
|
IDC |
ER – PR |
Neg. |
NA |
NA |
NA |
|
IDC |
ER – PR |
Pos. |
NA |
NA |
NA |
|
|
DCIS |
ER – PR |
NA |
NA |
NA |
NA |
|
|
DCIS |
ER – PR |
Neg. |
NA |
NA |
NA |
|
|
DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
|
IDC |
ER – PR |
Neg. |
NA |
NA |
NA |
|
|
Mele et al./2014 [25] |
IAC |
HER2 |
Pos. |
NA |
NA |
NA |
|
Limite et al./2014 [26] |
ACC (Ac) |
TN |
Neg. |
8 |
No |
No |
|
Kwon et al./2014 [27] |
DCIS, DCIS |
ER – PR, ER - PR |
NA, NA |
NA, NA |
NA |
NA |
|
Kılıç et al./2014 [28] |
DCIS |
NA |
NA |
NA |
NA |
NA |
|
Dandin et al./2014 [29] |
IDC - ILC - DCIS |
PR - HER2 |
Neg. |
6 |
No |
No |
|
Buteau et al./2014 [30] |
ILC |
NA |
Pos. |
NA |
No |
No |
|
Hayes et al./2013 [31] |
ILC |
ER |
Neg. |
NA |
NA |
NA |
|
Jahan et al./2012 [32] |
IDC, IDC |
NA, NA |
NA, NA |
NA, NA |
NA |
NA |
|
Butler et al./2012 [33] |
ILC - LCIS |
NA |
NA |
NA |
NA |
NA |
|
Ooe et al./2011 [34] |
DCIS |
ER – PR |
Neg. |
6 |
No |
No |
|
Lin et al./2011 [35] |
IDC - DCIS |
ER – PR |
NA |
24 |
No |
No |
|
Kato et al./2011 [36] |
DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
Abu-Rahmeh et al./ 2012 [37] |
IDC |
NA |
NA |
NA |
NA |
Yes |
|
Rao et al./ 2010 [38] |
IDC |
TN |
Pos. |
NA |
NA |
NA |
|
Petersson et al./2010 [39] |
IDC - DCIS |
ER – PR |
Neg. |
24 |
No |
No |
|
Tajima et al./2009 [40] |
ILC - LCIS |
ER |
NA |
NA |
NA |
NA |
|
Gashi-Luci et al./2009 [41] |
IDC - DCIS |
HER2 |
Neg. |
5 |
Yes |
NA |
|
Borecky et al./2008 [42] |
IDC - DCIS |
ER – PR |
Neg. |
NA |
NA |
NA |
|
IDC |
NA |
Neg. |
NA |
NA |
NA |
|
|
IDC - DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
|
Tiu et al./2006 [43] |
DCIS |
NA |
NA |
18 |
No |
No |
|
Shin et al./2006 [44] |
IDC - DCIS |
ER – PR |
Neg. |
16 |
No |
No |
|
Blanco et al./2005 [45] |
ACC (Ad) |
TN |
Neg. |
NA |
NA |
NA |
|
Abite et al./2005 [46] |
IDC |
NA |
NA |
NA |
NA |
NA |
|
Stafyla et al./2004 [47] |
LCIS |
NA |
NA |
24 |
No |
No |
|
Abe et al./ 2004 [48] |
IDC |
PR |
Pos. |
59 |
No |
No |
|
Adelekan et al./2003 [49] |
IC, LCIS - DCIS |
NA, NA |
NA, Pos. |
NA, NA |
NA |
No |
|
Yano et al./2001 [50] |
LCIS |
NA |
Neg. |
24 |
No |
No |
|
Gebrim et al./2000 [51] |
ILC |
NA |
Neg. |
NA |
No |
No |
|
Psarianos et al./1998 [52] |
DCIS |
NA |
NA |
NA |
NA |
NA |
|
Shah et al./ 1998 [53] |
LCIS |
NA |
NA |
25 |
No |
No |
|
Kurosum et al./1994 [54] |
IDC |
NA |
NA |
NA |
NA |
No |
|
Morimoto et al./1993 [55] |
LCIS |
NA |
NA |
132 |
No |
No |
|
Gupta et al./1992 [56] |
DCIS |
NA |
NA |
9 |
No |
No |
|
Gupta et al./1991 [57] |
CA |
NA |
Neg. |
10 |
No |
No |
|
Fukud et al./1989 [58] |
LCIS |
NA |
NA |
NA |
No |
No |
|
Yoshida et al./1985 [59] |
ILC |
ER |
Neg. |
32 |
No |
No |
|
Fond et al./1979 [60] |
DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
Konakry et a./1975 [61] |
LCIS |
NA |
Neg. |
60 |
No |
No |
|
LCIS |
NA |
Neg. |
36 |
No |
No |
|
|
LCIS |
NA |
Neg. |
36 |
No |
No |
|
|
LCIS |
NA |
Neg. |
24 |
No |
No |
|
|
LCIS |
NA |
Neg. |
NA |
No |
No |
|
|
Durso et al./1972 [62] |
LCIS |
NA |
NA |
NA |
NA |
NA |
| NA: non-available, DCIS: Ductal Carcinoma In Situ, IDC: Invasive Ductal Carcinoma, CIS: Carcinoma In Situ, IAC: Invasive apocrine LCIS - DCIScarcinoma, ILC: Invasive Lobular Carcinoma, LCIS: Lobular Carcinoma In Suspicious, , ACC (ac): Acinic Cell Carcinoma, ACC (Ad): Adenoid Cystic Carcinoma, IC: Invasive Carcinoma, CA: Carcinoma, ER: Estrogen Receptor, PR: Progesterone Receptor, HER2: Human Epidermal Growth Factor Receptor 2, TN: Triple Negative, HPE: Histopathological Examination, Pos.: positive, Neg.: negative, FU: Follow-up. | ||||||
Patients and tumor characteristics
The total number of patients was 72 females, with a mean age of 44.4 ± 13.6 years. Most patients presented with either a breast lump (43.1%) or a mass (30.5%), with a median presentation duration of 12 months. In 80.6% of cases, the disease was unilateral, with laterality distributed almost equally between the right side (42.1%) and the left (39.5%). The mean tumor size was 24.7 ± 13.3 millimeters. The past medical history was negative in 27.8% of cases, while seven cases (9.7%) had a positive history of other breast diseases, including breast mass in four cases and DCIS, fibrocystic breast disease, and IDC per case. The family history of breast cancer was positive in four cases (5.5%). On physical examination, information about the tumor surface was available for nine tumors (11.8%), all of which had a smooth surface. Of the 22 tumors with available data on consistency, 14 (63.6%) were firm, five (22.7%) were hard, and three (13.6%) were rubbery. Among the 28 tumors with existing mobility data, 25 (89.3%) were found to be mobile. Axillary lymphadenopathy was reported in four tumors (5.3%). On ultrasonography, 28 masses appeared benign (36.8%), and 20 cases were suspicious (26.3%), with ultrasonographic data unavailable for the remaining tumors (36.8%). Mammography data were available for 50 tumors, revealing 27 benign lesions (54%) and 23 suspicious lesions (46%). Among the 17 lesions with available magnetic resonance imaging (MRI) data, five were benign lesions (29.4%), and 12 were suspicious (70.6%). Core needle biopsy (CNB) or fine needle aspiration cytology (FNAC) revealed that 20 tumors (26.3%) were benign, 24 (31.6%) were malignant, and two (2.6%) were suspicious. The data on preoperative diagnosis was unavailable for 30 cases (39.5%). (Table 7).
|
Variables |
Frequency/ percentages |
|
Study design Case report Case series |
58 (95.0%) 3 (5.0 %) |
|
Country Japan USA Korea Brazil China Italy Taiwan Australia India New Zealand Singapore Turkey Others |
12 (19.7%) 9 (14.7%) 4 (6.6%) 3 (4.9%) 3 (4.9%) 3 (4.9%) 3 (4.9%) 2 (3.3%) 2 (3.3%) 2 (3.3%) 2 (3.3%) 2 (3.3%) 14 (22.9%) |
|
Age range (mean ± SD) |
18-80 (44.4 ± 13.6) |
|
Gender Female |
72 (100%) |
|
Presentation Lump Mass Incidental Nodule Nipple discharge N/A |
31 (43.1%) 22 (30.5%) 3 (4.1%) 2 (2.8%) 1 (1.4%) 13 (18.1%) |
|
Duration of presentation, median (Q1 - Q3), months |
12 (2-60) |
|
Laterality Unilateral Bilateral N/A |
58 (80.6%) 6 (8.3%) 8 (11.1%) |
|
Tumor location Right Left Bilateral N/A |
32 (42.1%) 30 (39.5%) 1 (1.3%) 13 (17.1%) |
|
Tumor size (mean ± SD), mm |
24.7 ± 13.3 |
|
PMH Nothing noteworthy Breast mass Hypertension CAH DCIS Fibrocystic breast disease IDC N/A |
20 (27.8%) 4 (5.5%) 1 (1.4%) 1 (1.4%) 1 (1.4%) 1 (1.4%) 1 (1.4%) 43 (59.7%) |
|
Family history of breast cancer Positive Negative N/A |
4 (5.5%) 31 (43.1%) 37 (51.4%) |
|
Surface of the mass Smooth N/A |
9 (11.8%) 67 (88.2%) |
|
Consistency of the mass Firm Hard Rubbery N/A |
14 (18.4%) 5 (6.6%) 3 (3.9%) 54 (71.1%) |
|
Mobility of the mass Mobile Non-mobile Partially fixed N/A |
25 (32.9%) 2 (2.6%) 1 (1.3%) 48 (63.2%) |
|
Axillary Lymphadenopathy Negative Positive N/A |
27 (35.5%) 4 (5.3%) 45 (59.2%) |
|
Radiological findings |
|
|
Ultrasonography Benign Suspicious N/A |
28 (36.8%) 20 (26.3%) 28 (36.8%) |
|
Mammography Benign Suspicious N/A |
27 (35.5%) 23 (30.3%) 26 (34.2%) |
|
Magnetic resonance imaging Suspicious Benign N/A |
12 (15.8%) 5 (6.6%) 59 (77.6%) |
|
Shape of the mass Oval Irregular Lobular Round Multilobulated N/A |
15 (19.7%) 2 (2.6%) 2 (2.6%) 2 (2.6%) 1 (1.3%) 54 (71.1%) |
|
Margin of the mass Well defined Irregular Ill-defined Lobulated Smooth Microlobulated Circumscribed N/A |
32 (42.1%) 7 (9.2%) 4 (5.3%) 3 (4%) 2 (2.6%) 1 (1.3%) 1 (1.3%) 26 (34.2%) |
|
Vascularity of the mass Yes No N/A |
8 (10.5%) 2 (2.6%) 66 (86.8%) |
|
Calcification Positive Negative N/A |
24 (31.6%) 11 (14.5%) 41 (53.9%) |
|
Cytology (CNB or FNAC) Benign Malignant (non-specified) DCIS IDC IDC – DCIS Suspicious IC ILC – LCIS Invasive apocrine carcinoma N/A |
20 (26.3%) 8 (10.5%) 7 (9.2%) 3 (4%) 3 (4%) 2 (2.6%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 30 (39.5%) |
|
Breast surgery Wide local excision Mastectomy Excisional biopsy N/A |
37 (50.7%) 24 (32.9%) 9 (12.3%) 3 (4.1%) |
|
Axillary surgery ALND SLNB None N/A |
17 (23.3%) 15 (20.6%) 29 (39.7%) 12 (16.4%) |
|
Chemotherapy Yes No NA |
11 (15.3%) 15 (20.8%) 46 (63.9%) |
|
Radiation therapy Yes No NA |
16 (22.2%) 14 (19.4%) 42 (58.3%) |
|
Hormonal therapy Yes No NA |
20 (27.8%) 2 (2.8%) 50 (69.4%) |
|
Post-operative HPE DCIS LCIS IDC IDC - DCIS ILC ILC - LCIS Carcinoma (non-specified) Acinic cell carcinoma Adenoid cystic carcinoma IDC - ILC - DCIS Invasive apocrine carcinoma LCIS – DCIS N/A |
23 (30.3%) 15 (19.7%) 15 (19.7%) 6 (7.9%) 5 (6.6%) 3 (4%) 3 (4%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) |
|
Immunohistochemistry ER – PR Triple-negative ER HER2 ER - PR - HER2 PR - HER2 PR N/A |
19 (25%) 8 (10.5%) 6 (7.9%) 4 (5.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 36 (47.4%) |
|
Axillary status Positive Negative N/A |
7 (9.2%) 32 (42.1%) 37 (48.7%) |
|
Follow-up, median (Q1-Q3), months |
24 (10-36) |
|
Recurrence No Yes N/A |
38 (52.8%) 1 (1.4%) 33 (45.8%) |
|
Metastasis No Yes N/A |
43 (59.7%) 2 (2.8%) 27 (37.5%) |
|
SD: Standard Deviation, N/A: non-available, CAH: Congenital Adrenal Hyperplasia, DCIS: Ductal Carcinoma In Situ, IDC: Invasive Ductal Carcinoma, CNB: Core Needle Biopsy, FNAC: Fine Needle Aspiration Cytology, CIS: Carcinoma In Situ, IC: Invasive Carcinoma, ILC: Invasive Lobular Carcinoma, LCIS: Lobular Carcinoma In Situ, ALND: Axillary Lymph Node Dissection, SLNB: Sentinel Lymph Node Biopsy, HPE: Histopathological Examination, ER: Estrogen Receptor, PR: Progesterone Receptor, HER2: Human Epidermal Growth Factor Receptor 2, Q1:first quartile, Q3: third quartile, PMH: past medical history. |
|
Management and outcome
The most commonly performed surgery was wide local excision (50.7%), followed by mastectomy (32.9%). Axillary lymph node dissection was carried out in 43.9% of cases. A total of 11 cases (15.3%) received chemotherapy, 16 cases (22.2%) underwent radiotherapy, and hormonal therapy was prescribed for 20 cases (27.8%). On histopathological examination, 11 tumors exhibited more than one pathology. DCIS was the most frequent finding (40.8%), followed by IDC (28.9%) and LCIS (28.4%). Immunohistochemical analysis showed that 20 out of 40 tumors (50%) were positive for both estrogen (ER) and progesterone receptors (PR). Of the 39 tumors with reported axillary status, 82.1% had negative axillary findings. The median follow-up period was 24 months, with quartile ranges of 10 to 36 months. Recurrence was observed in one case (1.4%), and metastasis occurred in two cases (2.8%) (Table 7).
Discussion
Carcinomas and high-risk lesions within fibroadenomas can either originate from the fibroadenoma itself and remain entirely encapsulated, or they can involve both the fibroadenoma and the adjacent breast tissue [2]. While rare, a small percentage of fibroadenomas may contain carcinomas or high-risk lesions, with reported incidence rates ranging from 0.002% to 0.125%. Fibroadenomas with malignant components are primarily found in patients 10 to 20 years older than the typical age for simple fibroadenomas [2]. In this systematic review, the mean age of affected patients was 44.4 years, further highlighting the trend of malignancies occurring in later decades of life.
The role of fibroadenomas as a potential risk factor for breast cancer is still not fully established [8]. It has been suggested that they may represent a long-term risk factor for breast cancer, particularly in women with complex fibroadenomas, proliferative disease, or a family history of breast cancer. Specifically, complex fibroadenomas are associated with a relative breast cancer risk that is approximately 3.10 times greater [6]. Another significant indicator of potential malignant transformation in fibroadenomas is the progressive mass size and thickness increase with advancing patient age [3]. A study has reported that the average tumor diameter of breast cancer occurring within a fibroadenoma is 2.46 cm [11]. Similarly, the mean tumor size in this systematic review was 2.47 ± 13.3 cm.
Frisch et al. reported that the predominant form of malignancy associated with breast cancer arising in fibroadenomas was CIS, with LCIS accounting for 66.9% and DCIS comprising 12.4%. Additionally, IDCs were more frequent among the invasive cases than ILCs [7]. Conversely, another study found that ductal and lobular carcinomas occur with equal frequency [6]. In this study, the distribution of malignancies within fibroadenomas revealed distinct differences from Frisch et al.’s study [7]. Notably, DCIS was the most frequent malignancy, accounting for 40.8% of tumors and LCIS represented 28.4% of tumors. The incidence of IDC was higher in this review at 28.9%, compared to 11% in the prior study [7]. Additionally, rarer malignancies like acinic cell carcinoma, adenoid cystic carcinoma, and invasive apocrine carcinoma were observed, suggesting a broader spectrum of tumor types associated with fibroadenomas than traditionally recognized.
The neoplastic proliferation of epithelial cells within the breast lobule characterizes LCIS. It is considered a precursor to ILC, similar to the relationship between DCIS and IDC. LCIS is now recognized as a general marker for breast cancer risk rather than a definitive pre-cancerous condition. It has been indicated that neoplasms within fibroadenomas behave similarly and have comparable prognoses to those occurring independently [9]. DCIS, also known as intraductal carcinoma, is a neoplasm that does not invade the basement membrane. This type of breast carcinoma develops within the ductal system, particularly in the terminal lobular duct unit. Although DCIS cannot metastasize and is considered non-lethal, its presence indicates an increased risk of developing invasive cancer if left untreated [8].
The preoperative diagnosis of malignant transformation within fibroadenoma is difficult and often necessitates surgical intervention for definitive confirmation [3]. This challenge stems from the overlap in clinical and radiological features between benign and malignant fibroadenomas, making it difficult to distinguish between the two preoperatively [4]. However, certain imaging characteristics can help identify carcinoma within fibroadenomas. Such malignancies tend to present with larger size, irregular shape, poorly defined margins, and abnormal calcifications, including linear, pleomorphic, or microcalcifications [12]. Sonographic evaluation of carcinomas within fibroadenomas typically reveals irregular lesions with indistinct borders. These tumors are often associated with marked hypoechoic shadowing, an echogenic halo, and distortion of surrounding tissue. Ultrasound is beneficial for tumor size assessment due to its high-resolution imaging capabilities. While mammography may reveal indistinct borders and microcalcifications, it is insufficient for diagnosing fibroadenomas with underlying carcinoma. Nonetheless, microcalcifications on mammography remain a valuable indicator of malignant transformation [3]. When calcifications are identified on mammography, ultrasound can be used to evaluate the invasiveness of the lesion and guide biopsy. Additionally, Doppler color imaging provides further insights into the internal vascularity of the tumor [13]. Dynamic MRI offers a reliable method for distinguishing malignant transformations from benign fibroadenomas by highlighting differences in vascularity. Benign fibroadenomas typically appear as round or oval masses with smooth margins on MRI, showing consistent enhancement into the late phase. In contrast, malignant lesions often display rapid early enhancement with variability in delayed enhancement, a hallmark of carcinoma [3]. Detecting malignant transformation can be particularly challenging, as clinical and radiological signs may remain masked until the tumor breaches the false capsule. As a result, definitive diagnosis is usually made through histopathological examination, emphasizing the importance of maintaining a high index of suspicion in these cases [3,4]. In the present study, of the 22 cases that reported tumor shape on imaging, 15 (68.2%) presented with an oval shape, while two cases (9.1%) showed an irregular shape. Tumor margins were well-defined in 32 out of 50 cases (64%), whereas seven (14%) exhibited irregular margins. Among the 10 cases reporting tumor vascularity, eight (80%) showed increased or high vascularity. Calcifications were observed in 24 out of 35 cases (68.6%) that provided data on this feature.
Common clinical techniques for obtaining pathological information include FNAC, hollow CNB, and mass excision biopsy. However, due to the inherent heterogeneity of these lesions, FNAC and CNB may not always provide conclusive results to definitively exclude malignancy in benign breast lesions that carry an increased risk of cancer development. Consequently, an open biopsy is recommended as a more reliable method for accurate diagnosis [15]. If imaging studies of a fibroadenoma indicate enlargement or any abnormal changes during follow-up examinations, it is essential to perform a CNB to ensure a definitive assessment. For patients aged 40 years and older with clinically benign fibroadenomas, clinicians should engage in discussions with these patients regarding the potential necessity of a CNB. This proactive approach allows for a thorough evaluation of changes and ensures appropriate diagnostic measures are implemented [12]. The diagnosis of fibroadenoma with carcinoma in the breast is contingent upon several critical criteria. Firstly, there must be clear evidence of epithelial heterogeneous hyperplasia or carcinoma within the fibroadenoma. Secondly, the cancerous tissue should remain confined to the capsule of the fibroadenoma, with only minimal focal infiltration into the surrounding breast tissue. Thirdly, it is crucial to exclude the possibility of infiltration from adjacent breast cancer into the fibroadenoma, as the coexistence of breast cancer and fibroadenoma does not qualify as intra-fibroadenoma carcinoma. Finally, the diagnosis must be supported by the results of immunohistochemical markers. These criteria facilitate a thorough and accurate assessment of fibroadenoma with carcinoma [15]. In this systematic review, pre-operative tissue biopsy using either CNB or FNAC was available for 46 tumors. Malignant features were observed in 24 tumors (52.2%), two tumors (4.3%) exhibited suspicious features, and 20 tumors (43.5%) were classified as benign. These findings highlight the importance of pre-operative biopsy and the challenges in accurately identifying the presence of malignancy in fibroadenomas.
Given the rarity of malignancy arising within fibroadenomas, standardized management guidelines are not well-established, leaving uncertainty as to whether these patients should be treated similarly to breast cancer patients or with a distinct approach. For benign fibroadenomas, lumpectomy remains the treatment of choice. However, if the tumor is close to or involves the resection margin, wider local excision may be necessary to ensure complete removal. Factors such as large tumor size, multifocality, and central breast location may also necessitate consideration of mastectomy [3,4,16]. If surgical margins are free of cancer, lumpectomy alone is often sufficient. The overall management strategy is dictated by the stage of the disease and the degree of metastasis, whether localized or distant. Conservative management, such as lumpectomy or wide local excision, is usually appropriate for small tumors. In cases of local metastasis, especially involving the axillary lymph nodes, axillary lymph node dissection is typically performed to ensure proper treatment [3]. Surgical intervention remains the definitive treatment and may be combined with radiotherapy or chemotherapy depending on individual case specifics [16]. In the current study, the most common procedure was wide local excision (50.7%), followed by mastectomy (32.9%). Excisional biopsy was performed in 12.3% of the cases. Axillary lymph node dissection was performed in 17 cases (23.3%), while sentinel lymph node biopsy was carried out in 15 cases (20.6%). Twenty-nine cases (39.7%) did not undergo axillary surgery. This variation in axillary management highlights the individualized approach to surgical treatment based on tumor characteristics, lymph node involvement, and disease progression.
The use of radiotherapy remains a topic of debate, with chemotherapy being the preferred treatment option in cases involving nodal metastasis. Some authors suggested that breast cancer arising within a fibroadenoma exhibits similar behavior to breast cancer at the same stage. Consequently, the treatment approach should align with standard breast cancer protocols, following similar therapeutic modalities [4,5,11,17]. The positive impact of radiation therapy on both survival rates and recurrence prevention when combined with lumpectomy has been reported. This approach is regarded as the standard of care for breast-conserving therapy in cases of DCIS and breast cancer. However, radiation therapy is not without drawbacks. It carries inherent risks, financial costs, and potential negative effects on patients' quality of life. Notably, long-term complications such as lung cancer and heart disease have been associated with breast cancer radiation therapy, particularly in patients who have a history of smoking [17]. Ni et al. stated that DCIS within a fibroadenoma is a heterogeneous condition with significant variability in local recurrence risks among patients. Consequently, the overall benefits of postoperative radiation therapy differ based on individual patient risk profiles. Low-risk patients who undergo breast-conserving surgery (BCS) without subsequent radiotherapy experience limited advantages from radiation. In contrast, high-risk patients show a greater benefit from the addition of radiotherapy. For instance, it has been revealed that patients treated with BCS alone had 8-year recurrence rates of 0%, 21.5%, and 32.1% for low-, intermediate-, and high-risk groups, respectively. This highlights the need for personalized treatment approaches based on risk stratification [15]. The current National Comprehensive Cancer Network (NCCN) guidelines recommend ER testing for patients with DCIS and advise considering tamoxifen for women with ER-positive disease, particularly those who undergo BCS without radiation. The goal is to optimize treatment outcomes and minimize the chances of cancer recurrence [7]. In this study, the data on chemotherapy was available for only 26 cases, of which 11 (42.3%) underwent chemotherapy as part of their treatment regimen. Additionally, among 30 cases with information on radiation therapy, 16 cases (53.3%) received the treatment regimen. Furthermore, 22 cases addressed hormonal therapy, and 20 (90.9%) indicated it was utilized in the treatment protocols.
Some scholars indicated that breast cancer developing within a fibroadenoma is generally associated with a more favorable prognosis compared to conventional breast cancer. This is primarily attributed to the higher incidence of hormone receptor (HR)-positive tumors in this subset, along with the frequent presentation of CIS and early-stage disease at diagnosis [12]. However, the prevalence of hormone receptor positivity in these cases may not significantly differ from that seen in typical breast cancer. ER positivity has been reported at 68.8%, and PR positivity at 62.5%, figures closely aligned with those observed in conventional breast cancer [11]. Despite these favorable characteristics, it has been indicated that approximately 10% of patients diagnosed with CIS within a fibroadenoma experience recurrence or metastasis, emphasizing the need for continued surveillance and individualized treatment strategies, even in cases with seemingly better prognostic indicators [3]. In this systematic review, among the 40 tumors with available hormone receptor status, six (15%) were HR-positive. The ER was positive in 26 tumors (65%), and PR was positive in 22 tumors (55%). The median follow-up duration was 24 months, during which one case (1.4%) reported recurrence, and two cases (2.8%) experienced metastasis. The primary limitation of this study is the lack of data on several variables in the reviewed studies, which may impact the generalizability of the findings.
Conclusion
Although rare, carcinomas arising within fibroadenomas may present considerable challenges in preoperative diagnosis, whether through imaging or cytology. Therefore, clinicians may find it necessary to approach fibroadenomas with increased caution.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: AMS, LRAP and SL were significant contributors to the conception of the study and the literature search for related studies. BAA, DAH, BOH, HAH, SHS, DAO, SSA and SMA were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. MGH, MNH and HOA were involved in the literature review, study design, and manuscript writing. YMM, HAH and SSO Literature review, final approval of the manuscript, and processing of the tables. HOA and AMS confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
Edoxaban and Cancer-Associated Venous Thromboembolism: A Meta-analysis of Clinical Trials
Fahmi H. Kakamad, Sami S. Omar, Farman J. Ahmed, Dana H. Mohammed Saeed, Rebaz M. Ali, Marwan N....
Abstract
Introduction
Cancer patients face a venous thromboembolism (VTE) risk that is up to 50 times higher compared to individuals without cancer. In 2010, direct oral anticoagulants (DOACs) such as apixaban, rivaroxaban, and edoxaban were introduced, consequently becoming the controversial oral anticoagulants for VTE therapy. This study is a meta-analysis of randomized clinical trials (RCTs) evaluating the use of edoxaban for treating VTE in cancer patients over different treatment durations.
Methods
Using Google Scholar, a systematic search for RCTs on edoxaban for cancer-associated VTE was performed. The data extracted covered patient numbers, age, gender, BMI, cancer type, edoxaban dosage, treatment duration, comorbidities, major bleeding, recurrent VTE incidence, and deaths. Statistical significance was set at 0.05.
Results
Out of 52 studies, nine with 3,190 cases met the inclusion criteria. The mean age was 66.68 years, with 1,604 females (50.28%). Major bleeding occurred in 192 patients (7.66%) in the 6- or 12-month group and 57 (8.35%) in the 3-month group (p=0.573). Recurrent VTE was observed in 145 patients (5.78%) in the 6- or 12-month group and 95 (13.91%) in the 3-month group (p<0.001). Deaths from any cause totaled 548 (21.86%) in the 6- or 12-month group and 165 (24.16%) in the 3-month group (p=0.110).
Conclusion
Cancer patients receiving edoxaban for six or 12 months experience a lower recurrence rate of VTE compared to those on a 3-month treatment. The incidence of major bleeding appears to be similar between the two treatment durations.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), frequently occurs in cancer patients. Cancer is a condition characterized by abnormal cells' uncontrolled proliferation and persistence. Those with VTE who have cancer are at a higher risk of experiencing recurrent thromboembolism [1, 2].
Patients with cancer face a risk of VTE that is up to 50 times greater compared to those without cancer. The annual incidence of VTE in cancer patients varies between 3% and 15% [3]. Risk factors for developing cancer-associated thrombosis (CAT) extend beyond large pelvic masses that compress the iliac veins. They also include comorbidities, immobilization, chemotherapy, targeted therapies (such as bevacizumab), surgeries like lymphadenectomy, and the presence of intravenous catheters. These factors can contribute to a prothrombotic or hypercoagulable state [4].
The VTE is linked with considerable morbidity and mortality. Therefore, it is crucial to start anticoagulant therapy promptly and to maintain it for as long as the patient remains at elevated risk for recurrent events [5]. Anticoagulant therapy poses challenges in cancer patients due to their increased risk of both recurrent VTE and major bleeding compared to those without cancer. These complications can interfere with cancer treatments [5].
Low-molecular-weight heparin (LMWH), unfractionated heparin (UFH), and vitamin K antagonists (VKA) have been utilized in the treatment of CAT. Major guidelines, including those from the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Thoracic Society, recommend LMWH for treating CAT [4]. Nonetheless, the effectiveness of LMWH beyond six months is not well established, and its use can be challenging due to the need for daily subcutaneous injections [4].
In 2010, direct oral anticoagulants (DOACs) like apixaban, rivaroxaban, and edoxaban were introduced. These DOACs, which include factor Xa and thrombin inhibitors, have minimal interactions with food or other medications, like LMWH. This property enables fixed dosing without the need for regular coagulation monitoring [1, 4].
Recent large randomized controlled trials (RCTs) have demonstrated the benefits of DOACs over conventional VKAs for treating VTE. Consequently, DOACs have become the preferred oral anticoagulant for VTE therapy [6,7]. Moreover, DOACs have been shown to be non-inferior to LMWH when treating CAT. As a result, the use of DOACs in CAT patients is on the rise [8,9].
The optimal duration of anticoagulation therapy is determined by weighing the risk of recurrent VTE if the treatment is discontinued against the risk of bleeding if it is continued. Factors such as patient preference, life expectancy, and cost should also be considered [5].
This study is a meta-analysis of RCTs assessing the use of edoxaban for VTE in cancer patients across various treatment durations.
Methods
Study design
This meta-analysis was conducted strictly following the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Data sources and search strategy
A meta-analysis was conducted using Google Scholar to identify all published randomized studies on edoxaban treatment for cancer-associated VTE. The search used the following keywords: (Edoxaban OR Lixiana OR cancer OR tumor OR malignancy OR malignant OR cancerous OR tumorous AND thromboembolism OR embolism OR thrombosis).
Eligibility criteria
In this meta-analysis, only RCT studies were included. Studies were excluded if they were 1) non-English, 2) only abstracts, or 3) did not meet the inclusion criteria. All references included in this study were assessed for eligibility [10].
Study selection and data extraction
The titles and abstracts of the identified studies were first screened, followed by a thorough full-text screening to determine eligibility. Various data were extracted from the included studies, including number of patients, age, gender, BMI, type of cancer, edoxaban dosage, treatment duration, comorbidities, history of major bleeding and VTE, incidence of major bleeding and recurrent VTE during Edoxaban treatment, and the number of deaths.
Statistical analyses
The data were first used in a qualitative synthesis and then quantitatively re-analyzed using the Chi-square test and Fisher's exact test with the Statistical Package for Social Sciences (SPSS) version 27.0. The level of statistical significance was set at 0.05.
Results
A total of 52 studies were found in the search. Sixteen were directly excluded due to duplication, non-English language, or being pre-prints. The remaining 36 studies were screened by reviewing their titles and abstracts, with none removed after screening. All 36 studies underwent full-text screening, where 27 were excluded for various reasons. The remaining studies were evaluated for eligibility (Fig. 1); ultimately, nine studies [3-5, 8, 11-15], comprising 3,190 cases, met the inclusion criteria (Table 1). The mean age of the patients was 66.68 ± 10.70 years, with a predominance of females, accounting for 1,604 (50.28%) of the total population. Among the comorbidities, hypertension was the most common, affecting 535 patients (16.77%), followed by diabetes in 208 patients (6.52%). A history of major bleeding or recurrent VTE was reported in 210 patients (6.58%). Gastrointestinal tract cancer was the most frequently mentioned type of cancer in the included studies, affecting 273 patients (8.56%).
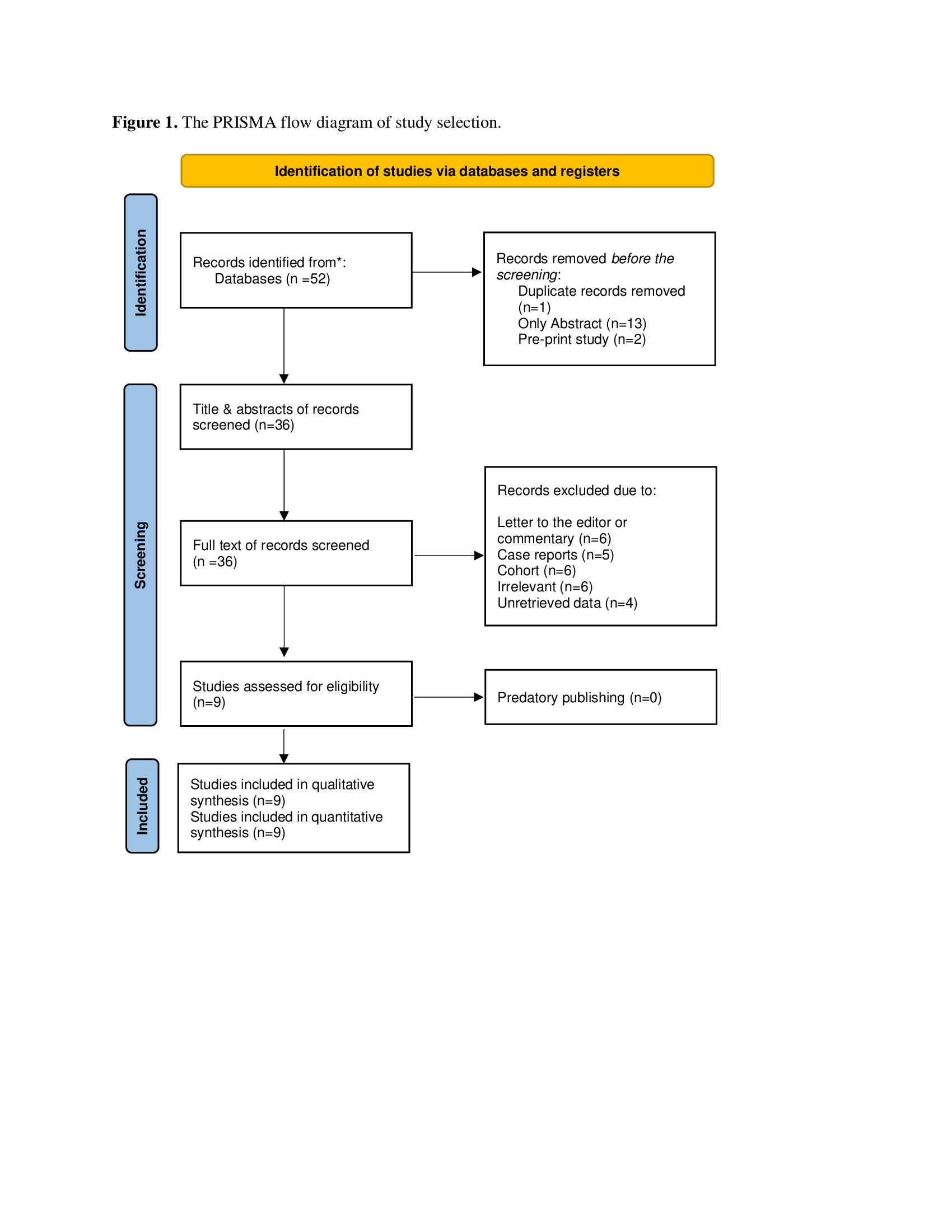
|
Author, year (Reference) |
Number of cases |
Study design |
Age (mean, SD) |
Gender (M/F) |
BMI (mean, SD) |
Dose |
Treatment period |
Outcome |
||||
|
60 mg |
30 mg |
3 mo |
≥ 6 mo |
Major Bleed |
Rec. VTE |
All causes Death |
||||||
|
Chatani et al. 2024 (11) |
151 |
RCT |
67.6 ± 9.8 |
81/70 |
26.7 ± 4.0 |
151 |
0 |
71 |
80 |
14 |
29 |
29 |
|
450 |
71.9 ± 9.7 |
86/364 |
21.1 ± 3.0 |
0 |
450 |
234 |
216 |
36 |
65 |
114 |
||
|
Chung et al. 2024 (12) |
20 |
RCT |
66.2±12.3 |
12/8 |
21.4±2.7 |
20 |
0 |
20 |
0 |
9 |
2 |
3 |
|
Di Nisio et al. 2019 (5) |
294 |
RCT |
64.4±11.1 |
156/ 138 |
N/A |
240 |
54 |
0 |
294 |
5 |
2 |
39 |
|
Mulder et al. 2020 (3) |
477 |
RCT |
N/A |
N/A |
N/A |
263 |
214 |
0 |
477 |
29 |
36 |
0 |
|
Nakamura et al. 2022 (13) |
53 |
RCT |
64.48±9.93 |
28/ 25 |
N/A |
21 |
31 |
53 |
0 |
4 |
3 |
3 |
|
Oride et al. 2023 (4) |
16 |
RCT |
60.2±10.5 |
0/16 |
22.1±3.90 |
3 |
13 |
0 |
16 |
1 |
2 |
0 |
|
Raskob et al. 2016 (14) |
378 |
RCT |
66.0 ± 13.0 |
181/197 |
N/A |
281 |
97 |
0 |
378 |
47 |
14 |
40 |
|
Raskob et al. 2018 (8) |
522 |
RCT |
64.3±11.0 |
277/ 245 |
N/A |
522 |
0 |
0 |
522 |
36 |
41 |
206 |
|
Yamashita et al. 2023 (15) |
296 |
RCT |
71.6±9.4 |
94/202 |
22.7±4.0 |
80 |
216 |
0 |
296 |
28 |
3 |
66 |
|
305 |
70.1±10.3 |
73/ 232 |
22.4±4.1 |
71 |
234 |
305 |
0 |
22 |
22 |
77 |
||
| RCT: randomized clinical trial, SD: standard deviation, M: male; F: Female, mg: milligram, mo: month, Rec: recurrent, VTE: venous thromboembolism, N/A: not applicable | ||||||||||||
Regarding Edoxaban dosage, the standard dose of 60 mg/day was administered to 1,812 patients (56.80%), while a reduced dose of 30 mg/day was given to 1,377 patients (43.17%). Only one patient received a 15 mg/day dose. The studies included in the review used three different treatment durations: 3 months, 6 months, or 12 months. A total of 2,507 patients, 78.59%, received edoxaban for either a 6-month or 12-month period, and 683 patients (21.41 %) received edoxaban for three months (Fig. 2). Major bleeding occurred in 192 patients (7.66 %) in the 6 or 12-month group and 57 patients (8.35%) in the 3-month group (p-value=0.573) (Table 2).
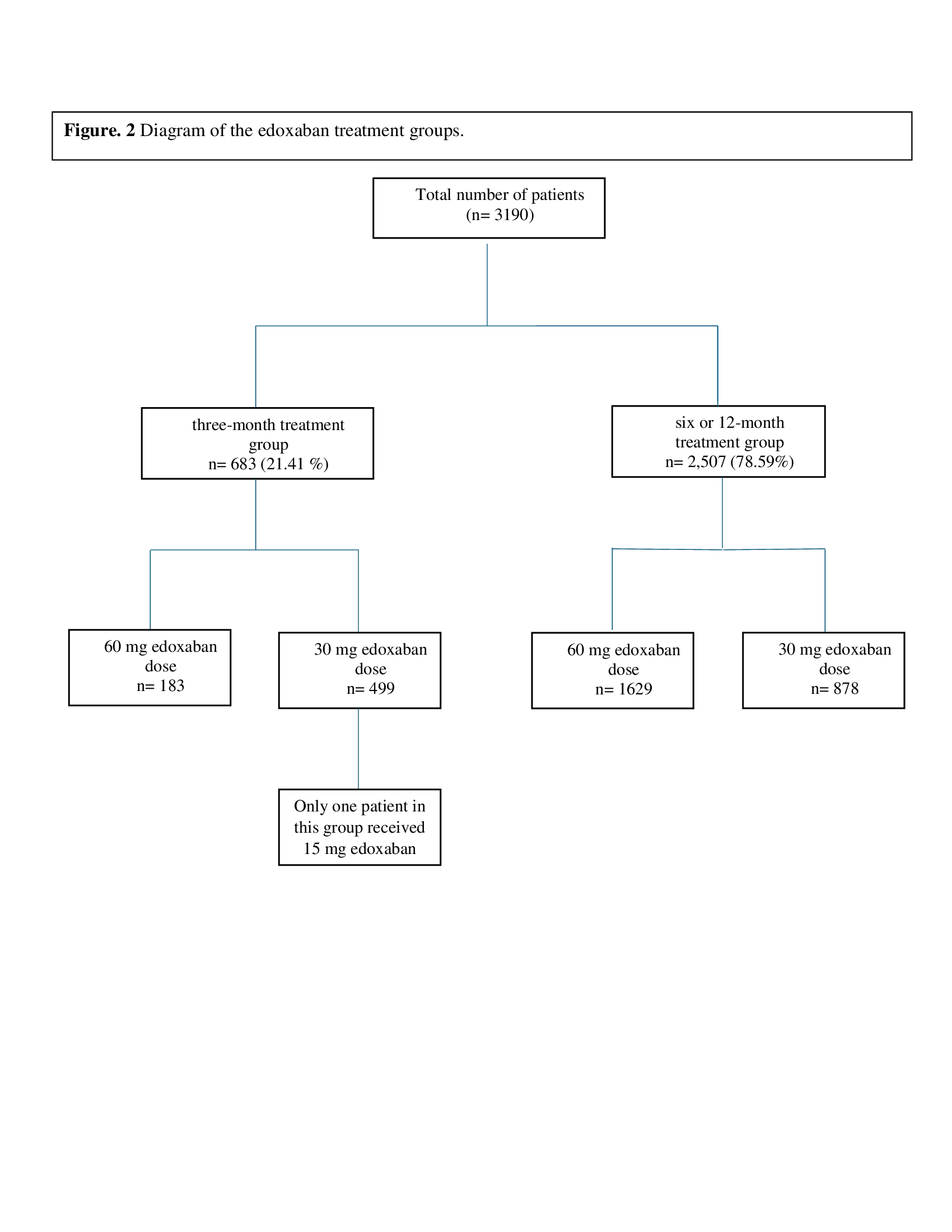
|
Variables |
Frequency/ percentages |
|
Number of total patients Age (mean of means) ± SD BMI (mean of means) ± SD a Body weight ≤60 kg, n (%) b |
3,190 (100%) 66.68 ± 10.70 22.73 ± 3.67 1,041 (32.63%) |
|
Gender, n (%) |
|
|
Female Male N/A |
1,604 (50.28 %) 1,109 (34.77%) 477 (14.95%) |
|
Hypertension, n (%) Yes No Unknown |
535 (16.77 %) 687 (21.54 %) 1,968 (61.69%) |
|
Diabetes mellitus, n (%) Yes No Unknown |
208 (6.52%) 1,014 (31.79%) 1,968 (61.69%) |
|
History of VTE, n (%) Yes No Unknown |
164 (5.14%) 2,082 (65.27%) 944 (29.59%) |
|
History of major bleeding, n (%) Yes No Unknown |
46 (1.44%) 1,156 (36.24%) 1,988 (62.32%) |
|
History of stroke, n (%) Yes No Unknown |
55 (1.72%) 1,167 (36.58%) 1,968(61.70%) |
|
Type of cancer, n (%) Gastrointestinal tract cancer Breast cancer Lung cancer Gynecological cancer Urogenital cancer Hematological malignancy Other known malignancies Unknown |
273 (8.56%) 134 (4.20%) 122 (3.83%) 105 (3.29%) 92 (2.88%) 79 (2.48%) 150 (4.70%) 2,235 (70.06%) |
|
Overall edoxaban dose group, n (%) 60 mg 30 mg 15 mg |
1,812 (56.80%) 1,377 (43.17%) 1 (0.03%) |
|
Treatment period, n (%) six or 12-month treatment 60 mg 30 mg |
2,507 (78.59%) 1629 (64.98%) 878 (35.02%) |
|
three-month treatment 60 mg 30 mg 15 mg |
683 (21.41 %) 183 (26.79%) 499 (73.06%) 1 (0.15%) |
|
Major bleeding during the treatment, n (%) six or 12-month treatment three-month treatment Overall |
192 (7.66%) 57 (8.35%) 249 (7.80%) |
|
Recurrence VTE, n (%) six or 12-month treatment three-month treatment Overall |
145 (5.78%) 95 (13.91%) 240 (7.52%) |
|
Number of deaths from all causes, n (%) six or 12-month treatment three-month treatment Overall |
548 (21.86%) 165 (24.16%) 713 (22.35%) |
|
a: The BMI belongs to 1,238 cases as it was provided in three studies, b: body weight ≤60 kg belongs to five of the included studies, HTN: hypertension, DM: diabetes mellitus, VTE: venous thromboembolism. |
|
Recurrent VTE was observed in 145 patients (5.78%) in the 6-month or 12-month group and 95 patients (13.91%) in the 3-month group (p-value<0.001). The number of deaths from any causes was 548 deaths (21.86%) in the 6-month or 12-month, followed by 165 deaths (24.16%) in the 3-month group (p-value=0.110) (Table 3).
|
Parameters |
6 or 12-month treatment |
3-month group |
p-value |
|
Major bleeding |
192 (7.66%) 2,315 (92.34) |
57 (8.35%) 626 (91.65) |
0.573 |
|
Recurrent VTE Yes No |
145 (5.78%) 2,362 (94.22%) |
95 (13.90%) 588 (86.10) |
<0.001 |
|
All causes of death Yes No |
548 (21.86%) 1,959 (78.14%) |
165 (24.16%) 518 (75.84%) |
0.110 |
Discussion
Thrombogenesis can be triggered by several factors, including age, a sedentary lifestyle, and advances in imaging technologies that have led to increased diagnosis rates. Other contributing factors include using hematopoietic agents, blood transfusions, invasive intravascular catheters, and the effects of new antineoplastic drugs [16].
The high incidence and recurrence of VTE in cancer patients is well-documented. The pathogenesis of cancer-associated coagulopathy is complex and involves several mechanisms [17]. Tumor cells can trigger blood coagulation through various processes, including producing procoagulant factors, fibrinolytic activities, and pro-aggregating effects [17]. The hemostatic side effects of oncological treatments, such as cytotoxic drugs, hormone therapy, anti-angiogenic therapy, radiotherapy, and surgery, also contribute to blood coagulopathies [18].
Treating VTE associated with active malignancy involves not only managing the thrombosis but also addressing the cancer itself. This approach is complicated by issues such as bleeding risks and the potential for recurrent VTE [19].
The LMWH has been the preferred anticoagulant for CAT following evidence from major RCTs that demonstrated its superiority over traditional vitamin K antagonists (VKAs) (20-23). Recently, several large RCTs have compared DOACs and LMWH, demonstrating that DOACs are non-inferior to LMWH for treating venous VTE in patients with CAT, and they have emerged as an alternative to LMWH [6,8,9].
Based on the results from several clinical trials, including Hokusai VTE Cancer (edoxaban vs. dalteparin, n=522 vs. 524), Caravaggio (apixaban vs. dalteparin, n=576 vs. 579), and SELECT-D (rivaroxaban vs. dalteparin, n=203 vs. 203), as well as meta-analyses incorporating these studies, DOACs are deemed effective, safe, and useful for treating CAT [6,8,9]. These trials indicated that the VTE recurrence rates with DOACs range from 4% to 8%, and the major bleeding rates range from 3% to 7%, which are comparable to those observed with LMWH (3). In line with the studies referenced, the present review found that the overall recurrence rate and major bleeding were 7.52% and 7.80%, respectively.
In a sub-analysis of the HOKUSAI trial, which included 771 cancer patients with VTE, edoxaban was associated with a trend toward lower VTE recurrence and significantly reduced clinically relevant bleeding compared to warfarin [14]. Similarly, other Xa inhibitors, such as rivaroxaban and apixaban, demonstrated comparable benefits in sub-analyses of the EINSTEIN-DVT and PE trials [24] and the AMPLIFY trial [6]. These findings suggest that Xa inhibitors may be as effective as, and potentially safer than, warfarin for treating VTE in cancer patients [17].
A recent RCT, the ONCO DVT study, demonstrated the potential benefits of extended anticoagulation therapy with edoxaban for patients with cancer-associated isolated distal DVT, particularly in reducing thrombotic risk [15]. However, concerns have been raised about the increased risk of bleeding associated with prolonged anticoagulation in patients with cancer-associated VTE. Therefore, individual risk assessment and tailored treatment strategies are crucial for optimizing management in these patients [11]. In their RCT, Chatani et al. found that the 12-month cumulative incidence of major bleeding was higher in the 12-month treatment group compared to the 3-month group for patients receiving 60 mg of edoxaban (14.3% vs. 4.4%, p-value = 0.046). However, for the 30 mg edoxaban subgroup, the incidence of major bleeding did not differ significantly between the two treatment durations (8.7% vs. 8.6%, p-value = 0.89). However, the cumulative 12-month incidence of symptomatic recurrent VTE was lower in the 12-month group compared to the 3-month group for both the 60 mg and 30 mg doses (1.1% vs. 7.6%, p-value = 0.002) [11]. In the current review, major bleeding occurred in 7.66% of patients in the 6 or 12-month treatment group and 8.35% of those in the 3-month group for both the 60 mg and 30 mg doses, with no statistically significant difference found (p-value = 0.573). Consistent with Chatani et al.’s findings, the recurrence rate of VTE was higher in the 3-month group for both doses.
In a RCT conducted by Raskob and colleagues, edoxaban was used for 522 cases of cancer-associated VTEs; the number of deaths from any cause in their study was 39.5% [8]. In the present review, the mortality rate from any cause was 22.35%. The comparison between the two groups for both the 60 mg and 30 mg doses showed no significant difference (p-value = 0.110).
Conclusion
Cancer patients receiving edoxaban for six or 12 months experience a lower recurrence rate of VTE compared to those on a 3-month treatment. The incidence of major bleeding appears to be similar between the two treatment durations.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: FHK, SSO and RMA were significant contributors to the conception of the study and the literature search for related studies. FJA, DHMS, MNH and KFH were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. HAN, NSS and MQM were involved in the literature review, study design, and manuscript writing. DAO, SHT, RJR, SHM and TNM Literature review, final approval of the manuscript, and processing of the tables. FHK and HAN confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
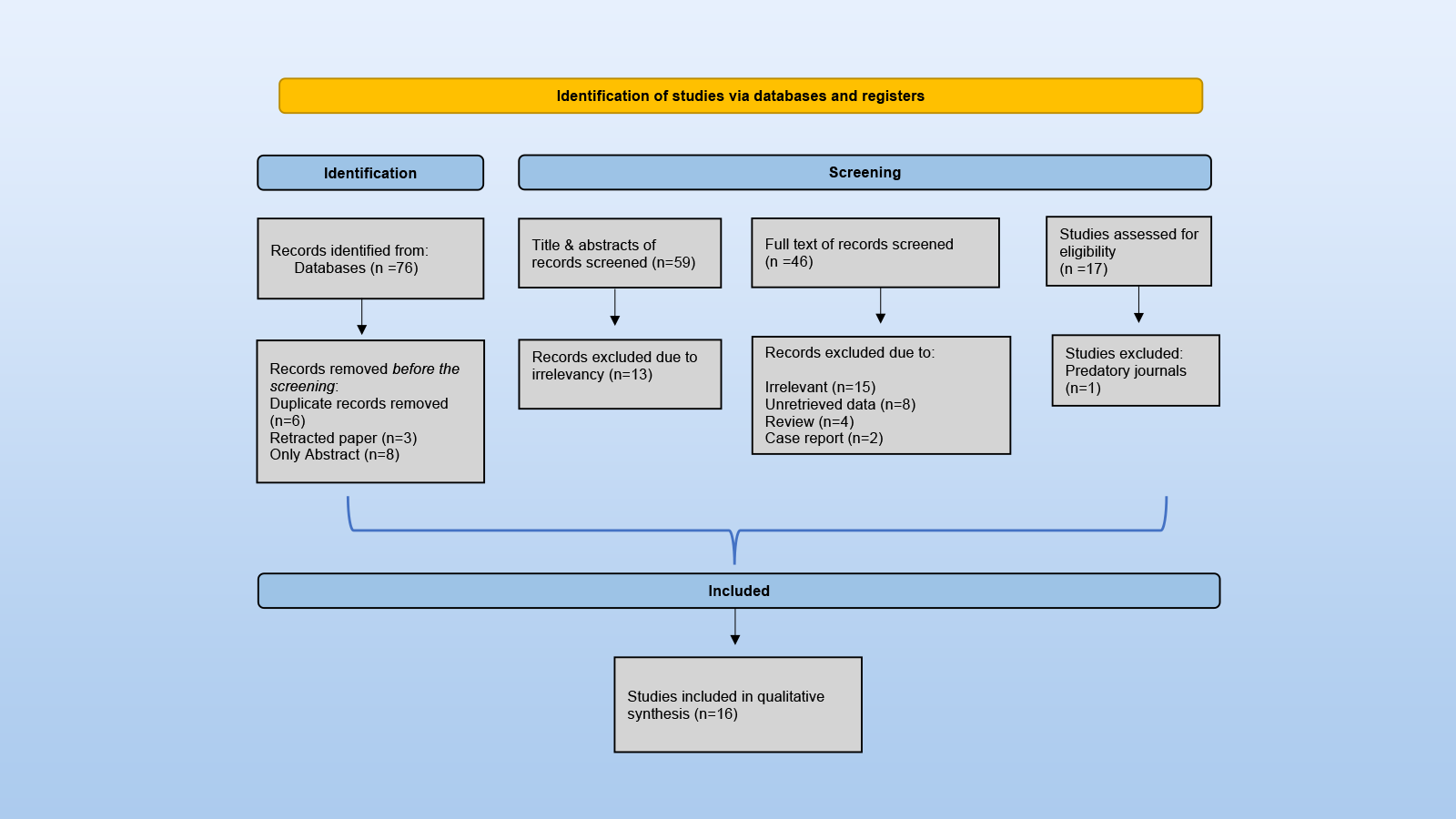
Microwave Ablation with or Without Chemotherapy in Management of Non-Small Cell Lung Cancer: A Systematic Review
Fahmi H. Kakamad, Rebaz M. Ali, Soran H. Tahir, Ameer M. Salih, Berun A. Abdalla, Lana RA....
Abstract
Introduction
Microwave ablation (MWA) has emerged as a minimally invasive treatment for patients with inoperable non-small cell lung cancer (NSCLC). However, whether it is more effective as a standalone treatment or in combination with chemotherapy warrants further investigation. This systematic review assesses the efficacy and safety of MWA as a standalone treatment and in combination with chemotherapy in managing NSCLC.
Methods
Studies were included if MWA was used either as a standalone treatment or combined with chemotherapy for managing NSCLC, regardless of whether chemotherapy was administered before or after MWA.
Results
The patient cohort included 928 patients. In 63.8% of the cases, MWA was used alone, and in 36.2% with chemotherapy. Complications from MWA alone were higher (59.29% vs. 32.74%). The tumor stage in 52.36% of the cases who underwent MWA alone was stage I; however, it was the IV stage in 82.44% of the cases who underwent MWA combined with chemotherapy. Patients with available data and treated with MWA alone experienced higher local progression (26% vs. 18.5%), distant recurrence (51.5% vs. 38.5%), and both local and distant recurrence (10.8% vs. 2.6%). Reported complete response was 88.6% among cases that underwent MWA alone. While it was 78.0% in those who underwent combined MWA and chemotherapy. The median overall survival was higher in the MWA alone group (24.9 to 69.6 months vs. 21.3 to 23.90 months).
Conclusion
MWA combined with chemotherapy may represent a more effective option, with a slightly similar treatment response, reducing the risk of recurrence and minimizing complications.
Introduction
Lung cancer is the leading cause of cancer-related mortality among both men and women worldwide [1,2]. It primarily consists of two main types: non-small cell lung cancer (NSCLC), which comprises 85% of cases, and small cell lung cancer (15%). The World Health Organization classifies NSCLC into three main subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [1]. NSCLC has one of the lowest five-year survival rates, hovering around 20% [3]. Cigarette smoking is the primary risk factor for lung cancer, responsible for 85% to 90% of cases. The risk is closely linked to smoking extent and exposure to carcinogens like asbestos, ionizing radiation, environmental toxins, and certain metals. Other risk factors include pulmonary fibrosis and alcohol consumption [1]. Occupational exposures, particularly to carcinogens like crystalline silica, asbestos, and radioactive materials, significantly increase lung cancer risk. In addition, familial clustering suggests a hereditary component to the disease [4]. Although smoking remains the leading cause, 12% of lung cancer cases are non-smokers, with higher rates in women [1,5]. The most common symptom of the disease is cough, followed by hemoptysis and chest pain [6]. Lung cancer is often diagnosed at intermediate or advanced stages due to its low early diagnostic rate, high malignancy, and complex biological characteristics [7]. However, low-dose computed tomography screening has improved early detection rates [8]. Surgical resection is the preferred treatment for early-stage NSCLC when the disease is operable. However, despite advancements in sub-lobar resection techniques to preserve lung function, more than 25% of patients with early-stage NSCLC are unable to undergo surgery due to factors like poor cardiopulmonary function, anatomical challenges, failure of conventional therapies, or personal choice. For these patients, non-surgical options such as stereotactic ablative radiotherapy, targeted therapies, and thermal ablation are typically recommended [6,8-10]. Thermal ablation therapies use various approaches to destroy cancer cells through heat or cold. In recent years, microwave ablation (MWA) has emerged as a viable alternative, offering outcomes comparable to lobectomy [8,9]. To our knowledge, no systematic review has been conducted on the effect of MWA alone or in combination with chemotherapy in managing NSCLC. This systematic review aims to evaluate the efficacy and safety of both treatment regimens in managing NSCLC.
Methods
Study design
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and search strategy
A systematic search on PubMed and Google Scholar was conducted to identify relevant English-language studies using MWA with chemotherapy or MWA alone in treating NSCLC. The search utilized the keywords "microwave ablation," "chemotherapy," "non-small cell lung cancer," "adenocarcinoma," "squamous cell carcinoma," and "large cell carcinoma."
Eligibility criteria
Studies were included if MWA was used either as a standalone treatment or combined with chemotherapy for managing NSCLC, regardless of whether chemotherapy was administered before or after MWA. Excluded studies included abstracts, retracted papers, case reports, reviews, and publications in predatory journals [11].
Study selection and data extraction
The following data were extracted from each eligible article: author, year of publication, study design, sample size, patient demography, tumor characteristics, management, characteristics of the MWA (frequency, antenna length, anesthesia type, power, ablation time), the chemotherapy drug, complications, and the outcomes.
Statistical analysis
The extracted data were collected in a Microsoft Excel sheet (2021) and then transferred into the Statistical Package for the Social Sciences software (version 27). Qualitative analysis was conducted, and the data were presented as frequency, percentage, mean with standard deviation, and median with range.
Results
Study selection
A total of 76 studies were identified through the search. Before screening, 17 studies were excluded due to duplication (n=6), retraction (n=3), and being available only as abstracts (n=8), leaving 59 studies for title and abstract screening. At this stage, 13 irrelevant studies were excluded. Irrelevant studies were those utilizing treatment modalities other than MWA with or without chemotherapy or where patients received other treatments before or after the primary intervention. Consequently, 46 studies underwent full-text screening, which led to the exclusion of 29 studies due to irrelevancy (n=15), unretrievable data (n=8), review articles (n=4), and case reports (n=2). Then, another study was excluded for being published in a predatory journal. Ultimately, 16 studies involving 928 cases met the eligibility criteria and were included [7-9,12-24] (Figure 1). The majority of included studies were cohort studies (n=14), along with two randomized controlled trials (RCTs) (Tables 1 and 2).
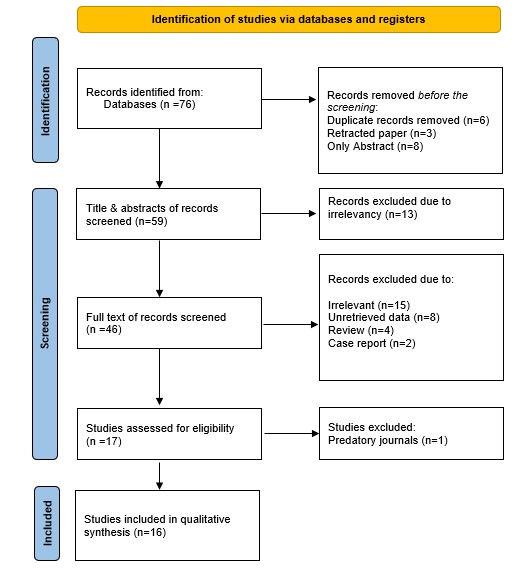
|
Author, year [Reference] |
Study design |
No. of cases |
Mean age) |
Gender |
Mean tumor size (cm) |
Tumor staging |
Tumor type |
Location (Lobe) |
Management |
Guidance |
Frequency (MHz) |
Antenna length (Max, mm) |
Antenna diameter (Max, G) |
Active tip (mm) |
Anesthesia |
||||||||||
|
M |
F |
I |
II |
III |
IV |
ADC |
SCC |
Other |
U&M |
L |
CS |
LA |
LA + CS |
LA + IV |
|||||||||||
|
Shan et al. 2021 [7] |
RCT |
67 |
61.5 |
46 |
21 |
3.8 |
0 |
0 |
0 |
67 |
29 |
38 |
N/A |
N/A |
N/A |
MWA + Chemo. |
CT |
N/A |
N/A |
N/A |
N/A |
0 |
67 |
0 |
0 |
|
Wu et al. 2024 [8] |
Cohort |
55 |
59.75 |
40 |
15 |
2.89 |
55 |
0 |
0 |
0 |
28 |
23 |
4 |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
19 |
N/A |
55 |
0 |
0 |
0 |
|
Han et al. 2019 [9] |
Cohort |
63# |
82.1 |
40 |
23 |
N/A |
N/A |
N/A |
N/A |
N/A |
47 |
17 |
1 |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
18 |
15 |
0 |
63 |
0 |
0 |
|
Lv et al. 2023 [12] |
Cohort |
118 |
N/A |
69 |
49 |
N/A |
N/A |
N/A |
N/A |
N/A |
94 |
N/A |
N/A |
73 |
45 |
MWA |
CT |
2450 |
180 |
18 |
N/A |
0 |
118 |
0 |
0 |
|
Xu et al. 2023 [13] |
Cohort |
33* |
68.4 |
19 |
14 |
4.4 |
6 |
3 |
7 |
1 |
25 |
7 |
1 |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
18 |
15 |
0 |
0 |
0 |
33 |
|
Li et al. 2023 [14] |
Cohort |
19 |
71.42 |
15 |
4 |
2.06 |
19 |
0 |
0 |
0 |
6 |
12 |
1 |
14 |
5 |
MWA |
CT |
2450 |
180 |
18 |
5 |
0 |
19 |
0 |
0 |
|
Hu et al. 2021 [15] |
Cohort |
68 |
83.1 |
44 |
24 |
2.3 |
68 |
0 |
0 |
0 |
41 |
24 |
N/A |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
17 |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Das et al. 2019 [16] |
Cohort |
56 |
59.1 |
34 |
22 |
2.9 |
0 |
0 |
32 |
24 |
43 |
10 |
3 |
37 |
19 |
MWA |
CT |
N/A |
N/A |
20 |
N/A |
0 |
56 |
0 |
0 |
|
Wei et al. 2019 [17]** |
Cohort |
18 |
74 |
9 |
9 |
3.3 |
0 |
0 |
8 |
10 |
13 |
N/A |
N/A |
10 |
8 |
MWA |
CT |
2450 |
180 |
20 |
N/A |
0 |
18 |
0 |
0 |
|
Wei et al. 2019 [17]** |
Cohort |
36 |
76 |
21 |
15 |
4.3 |
0 |
0 |
16 |
20 |
28 |
N/A |
N/A |
24 |
12 |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
36 |
0 |
0 |
|
Wang et al. 2018 [18] |
Cohort |
46 |
N/A |
22 |
24 |
N/A |
46 |
0 |
0 |
0 |
18 |
21 |
7 |
N/A |
N/A |
MWA |
CT |
N/A |
200 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Yao et al. 2018 [19] |
Cohort |
54 |
56.65 |
37 |
17 |
3.01 |
54 |
0 |
0 |
0 |
27 |
16 |
11 |
30 |
24 |
MWA |
CT |
N/A |
N/A |
N/A |
N/A |
54 |
0 |
0 |
0 |
|
Yang et al. 2014 [20] |
Cohort |
47 |
69.4 |
30 |
17 |
N/A |
47 |
0 |
0 |
0 |
28 |
13 |
N/A |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
20 |
15 |
0 |
47 |
0 |
0 |
|
Liu et al. 2013 [21] |
Cohort |
15 |
71.25 |
11 |
4 |
2.55 |
15 |
0 |
0 |
0 |
N/A |
N/A |
N/A |
10 |
5 |
MWA |
CT |
2450 |
140 |
N/A |
16 |
0 |
0 |
15 |
0 |
|
Wei et al. 2020 [22] |
RCT |
148 |
59 |
96 |
52 |
3.6 |
0 |
0 |
31 |
117 |
116 |
N/A |
N/A |
N/A |
N/A |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
148 |
0 |
0 |
|
Wei et al. 2015 [23] |
Cohort |
46 |
58.5 |
27 |
19 |
3.7 |
0 |
0 |
8 |
38 |
36 |
N/A |
N/A |
32 |
14 |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
46 |
0 |
0 |
|
Wei et al. 2014 [24] |
Cohort |
39 |
57 |
22 |
17 |
3.84 |
0 |
0 |
4 |
35 |
27 |
N/A |
N/A |
28 |
11 |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
39 |
0 |
0 |
|
Author, year [Reference] |
Power (Max, W) |
Ablation time (Max, Min) |
Ablation beyond margin (max, mm) |
Chemotherapy drug |
Complications |
Outcome |
OS (median, month) |
PFS (median, month) |
follow up (median, month) |
D |
A |
||||||||||||
|
Pem |
Doc |
Pac |
Gem |
Tig |
Cis |
Carbo |
Neda |
PTX |
PE |
Other |
LP |
R |
CR |
PR |
|||||||||
|
Shan et al. 2021 [7] |
80 |
20 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
6 |
N/A |
36 |
N/A |
N/A |
8 |
28 |
N/A |
4.5 |
6 |
N/A |
N/A |
|
Wu et al. 2024 [8] |
40 |
N/A |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
18 |
2 |
27 |
N/A |
31 |
N/A |
N/A |
69.6 |
N/A |
55.2 |
N/A |
N/A |
|
Han et al. 2019 [9] |
80 |
20 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
15 |
2 |
7 |
14 |
6 |
N/A |
N/A |
50.0 |
N/A |
21 |
N/A |
N/A |
|
Lv et al. 2023 [12] |
70 |
N/A |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
47 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Xu et al. 2023 [13] |
40 |
16.9 |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
4 |
4 |
15 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Li et al. 2023 [14] |
40 |
15 |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
5 |
2 |
18 |
N/A |
7 |
N/A |
N/A |
25 |
N/A |
20.4 |
14 |
5 |
|
Hu et al. 2021 [15] |
40 |
8 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
16 |
2 |
N/A |
N/A |
23 |
N/A |
N/A |
N/A |
N/A |
45 |
20 |
48 |
|
Das et al. 2019 [16] |
80 |
10 |
5 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
23 |
14 |
36 |
15 |
48 |
N/A |
N/A |
27.5 |
11.0 |
19.5 |
N/A |
N/A |
|
Wei et al. 2019 [17]** |
70 |
N/A |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
15 |
8 |
N/A |
N/A |
N/A |
15 |
3 |
24.9 |
14.1 |
N/A |
N/A |
N/A |
|
Wei et al. 2019 [17]** |
100 |
33 |
10 |
23 |
2 |
5 |
3 |
3 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
27 |
9 |
21.6 |
4.8 |
N/A |
N/A |
N/A |
|
Wang et al. 2018 [18] |
N/A |
N/A |
5 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
7 |
4 |
N/A |
N/A |
N/A |
46 |
N/A |
32.7 |
N/A |
N/A |
N/A |
N/A |
|
Yao et al. 2018 [19] |
N/A |
10 |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
7 |
N/A |
11 |
N/A |
39 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Yang et al. 2014 [20] |
N/A |
8 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
30 |
16 |
42 |
13 |
13 |
N/A |
N/A |
33.8 |
N/A |
30 |
26 |
21 |
|
Liu et al. 2013 [21] |
110 |
7 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
1 |
5 |
N/A |
9 |
2 |
N/A |
N/A |
12 |
N/A |
N/A |
|
Wei et al. 2020 [22] |
100 |
8 |
10 |
97 |
23 |
8 |
20 |
N/A |
40 |
19 |
89 |
N/A |
N/A |
N/A |
27 |
N/A |
132 |
N/A |
N/A |
10.3 |
13.1 |
N/A |
N/A |
|
Wei et al. 2015 [23] |
80 |
N/A |
5 |
19 |
16 |
4 |
7 |
N/A |
N/A |
N/A |
N/A |
18 |
15 |
9 |
9 |
N/A |
39 |
7 |
23.9 |
10.9 |
21 |
16 |
30 |
|
Wei et al. 2014 [24] |
70 |
11 |
5 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
12 |
7 |
7 |
7 |
16 |
N/A |
33 |
21.3 |
8.6 |
11.2 |
9 |
30 |
|
RCT: Randomized clinical trials, N/A: Not available, M: Male, F: Female, ADC: Adenocarcinoma, SCC: Squamous cell carcinoma, U: Upper, M: Middle, L: Lower, MWA: Microwave ablation, Chemo.: Chemotherapy, CT: Computed tomography, MHz: Megahertz, Max: Maximum, mm: millimeter, G: Gauge, CS: Conscious sedation, LA: Local anesthesia, IV: Intravenous, W: Watt, Min: Minute, Pem: Pemetrexed, Doc: Docetaxel, Pac: Paclitaxel, Gem: Gemcitabine, Tig: Tigio, Cis: Cisplatin, Carbo: Carboplatin, Neda: Nedaplatin, PTX: Pneumothorax, PE: Pleural effusion, LP: Local progression, R: Recurrence, CR: Complete response, PR: Partial response, OS: Overall survival, PFS: Progression-free survival, D: Death, A: Alive. *Tumor staging was available for only 17 cases. #For 63 cases, 65 tumors were diagnosed. **The same study with two arms, one with only MWA and the other with MWA combined chemotherapy. |
|||||||||||||||||||||||
Patients and Tumor Characteristics
The total sample size was 928 cases, with a gender distribution of 582 males (62.70%) and 346 females (37.30%). The mean age of the patients was 66.80 ± 8.87 years, and the mean tumor size was 3.33 ± 0.73 cm. Most of the cases were in stage IV (33.62%) and stage I (33.41%). Adenocarcinoma was the most common tumor type (65.52%), followed by squamous cell carcinoma (19.50%). The tumors were commonly located in the upper and middle lobes of the lungs (27.80%) (Table 3).
|
Variables |
Frequency & Percentages |
||
|
Overall |
MWA alone group |
MWA plus Chemotherapy group |
|
|
No. of cases |
928 |
592 (63.80%) |
336 (36.20%) |
|
Age (mean ± SD, year) |
66.80 ± 8.87 |
69.52 ± 9.07 |
62.28 ± 6.96 |
|
Study design Cohort Randomized clinical trial |
15 (88%) 2 (12%) |
12 (80%) 0 (0%) |
3 (20%) 2 (100%) |
|
Gender Male Female |
582 (62.70%) 346 (37. 30%) |
370 (62.50%) 222 (37.50%) |
212 (63.10%) 124 (36.90%) |
|
Tumor Size (mean ± SD, cm) |
3.33 ± 0.73 |
2.93 ± 0.72 |
3.86 ± 0.24 |
|
Tumor stage I II III IV N/A |
310 (33.41%) 3 (0.32%) 106 (11.42%) 312 (33.62%) 197 (21.23%) |
310 (52.36%) 3 (0.51%) 47 (7.94%) 35 (5.91%) 197 (33.28%) |
0 (0.00%) 0 (0.00%) 59 (17.56%) 277 (82.44%) 0 (0.00%) |
|
Tumor type Adenocarcinoma Squamous cell carcinoma Adenosquamous carcinoma Bronchoalveolar carcinoma Large cell neuroendocrine carcinoma Large cell carcinoma N/A |
608 (65.52%) 181 (19.50%) 7 (0.75%) 8 (0.86%) 1 (0.11%) 12 (1.29%) 111 (11.97%) |
372 (62.84%) 143 (24.16%) 7 (1.18%) 8 (1.35%) 1 (0.17%) 12 (2.03%) 49 (8.27%) |
236 (70.24%) 38 (11.31%) 0 (0.00%) 0 (0.00%) 0 (0.00%) 0 (0.00%) 62 (18.45%) |
|
Location (Lobe) Upper and middle Lower N/A |
258 (27.80%) 143 (15.41%) 527 (56.79%) |
174 (29.39%) 106 (17.91%) 312 (52.70%) |
84 (25.00%) 37 (11.01%) 215 (63.99%) |
|
Management MWA MWA + Chemotherapy |
592 (63.79%) 336 (36.21%) |
592 (100%) 0 (0%) |
0 (0%) 336 (100%) |
|
MWA characteristics |
|
|
|
|
Guidance CT scan |
928 (100%) |
592 (100%) |
336 (100%) |
|
Frequency (MHz) 2450 N/A |
705 (75.97%) 223 (24.03%) |
436 (73.65%) 156 (26.35%) |
269 (80.06%) 67 (19.94%) |
|
Antenna length (Max, mm) 200 180 140 N/A |
46 (4.96%) 690 (74.35%) 15 (1.62%) 177 (19.07%) |
46 (7.77%) 421 (71.11%) 15 (2.53%) 110 (18.59%) |
0 (0.00%) 269 (80.06%) 0 (0.00%) 67 (19.94%) |
|
Antenna diameter (Max, G) 20 19 18 17 N/A |
390 (42.03%) 55 (5.93%) 233 (25.11%) 68 (7.33%) 182 (19.60%) |
121 (20.44%) 55 (9.29%) 233 (39.36%) 68 (11.49%) 115 (19.42%) |
269 (80.06%) 0 (0.00%) 0 (0.00%) 0 (0.00%) 67 (19.94%) |
|
Active tip (mm) 16 15 5 N/A |
15 (1.62%) 143 (15.41%) 19 (2.05%) 751 (80.92%) |
15 (2.53%) 143 (24.16%) 19 (3.21%) 415 (70.10%) |
0 (0.00%) 0 (0.00%) 0 (0.00%) 336 (100.00%) |
|
Anesthesia type Conscious sedation Local anesthesia Local anesthesia + Conscious sedation Local anesthesia + intravenous N/A |
109 (11.75%) 657 (70.80%) 15 (1.62%) 33 (3.56%) 114 (12.27%) |
109 (18.41%) 321 (54.22%) 15 (2.53%) 33 (5.57%) 114 (19.27%) |
0 (0.00%) 336 (100.00%) 0 (0.00%) 0 (0.00%) 0 (0.00%) |
|
Power (Mean, W), 110 100 80 70 60 40 N/A |
71.76 ± 21.86 15 (1.62%) 184 (19.83%) 279 (30.06%) 175 (18.86%) 54 (5.82%) 175 (18.86%) 46 (4.95%) |
64.55 ± 22.96 15 (2.53%) 0 (0.00%) 166 (28.04%) 136 (22.97%) 54 (9.12%) 175 (29.56%) 46 (7.78%) |
85.00 ± 12.25 0 (0.00%) 184 (54.76%) 113 (33.63%) 39 (11.61%) 0 (0.00%) 0 (0.00%) 0 (0.00%) |
|
Ablation time (mean ± SD, max, min) |
14.38 ± 7.50 |
11.86 ± 4.80 |
18.40 ± 9.76 |
|
Ablation beyond margin (max, mm) 10 5 N/A |
481 (51.83%) 187 (20.15%) 260 (28.02%) |
297 (50.17%) 102 (17.23%) 193 (32.60%) |
184 (54.76%) 85 (25.30%) 67 (19.94%) |
|
Chemotherapy drug (for cases with available data) Pemetrexed Docetaxel Paclitaxel Gemcitabine Tigio Cisplatin Carboplatin Nedaplatin |
139 (14.98%) 41 (4.42%) 17 (1.83%) 30 (3.23%) 3 (0.32%) 40 (4.31%) 19 (2.05%) 89 (9.59%) |
- - - - - - - - |
139 (41.37%) 41 (12.20%) 17 (5.06%) 30 (8.93%) 3 (0.89%) 40 (11.90%) 19 (5.65%) 89 (26.49%) |
|
Complications (total number) PTX PE Emphysema Hemoptysis Pneumonia Pulmonary hemorrhage Pain Fever Infection Pulmonary abscess |
461 (49.68%) 176 (18.97%) 76 (8.19%) 16 (1.72%) 48 (5.17%) 14 (1.51%) 21 (2.26%) 60 (6.47%) 22 (2.37%) 27 (2.91%) 1 (0.11%) |
351 (59.29%) 140 (23.65%) 54 (9.12%) 16 (2.70%) 31 (5.24%) 14 (2.36%) 21 (3.55%) 44 (7.43%) 19 (3.21%) 11 (1.86%) 1 (0.17%) |
110 (32.74%) 36 (10.71%) 22 (6.55%) 0 (0.00%) 17 (5.06%) 0 (0.00%) 0 (0.00%) 16 (4.76%) 3 (0.89%) 16 (4.76%) 0 (0.00%) |
|
Local progression YES NO N/A |
90 (9.70%) 324 (34.91%) 514 (55.39%) |
47 (7.94%) 134 (22.64%) 411 (69.42%) |
43 (12.80%) 190 (56.55%) 103 (30.65%) |
|
Local recurrence YES NO N/A |
87 (9.38%) 269 (28.99%) 572 (61.63%) |
87 (14.70%) 269 (45.44%) 236 (39.86%) |
0 (0.00%) 0 (0.00%) 336 (100%) |
|
Distant recurrence YES NO N/A |
245 (26.40%) 241 (25.97%) 442 (47.63%) |
230 (38.85%) 217 (36.66%) 145 (24.49%) |
15 (4.46%) 24 (7.14%) 297 (88.40%) |
|
Local recurrence + Distant recurrence YES NO N/A |
13 (1.40%) 137 (14.76%) 778 (83.84%) |
12 (2.03%) 99 (16.72%) 481 (81.25%) |
1 (0.30%) 38 (11.30%) 297 (88.40%) |
|
Complete response YES NO N/A |
276 (29.74%) 67 (7.22%) 585 (63.04%) |
70 (11.82%) 9 (1.52%) 513 (86.66%) |
206 (61.31%) 58 (17.26%) 72 (21.43%) |
|
Partial response YES NO N/A |
82 (8.84%) 139 (14.98%) 707 (76.18%) |
5 (0.84%) 28 (4.73%) 559 (94.43%) |
77 (22.92%) 111 (33.04%) 148 (44.04%) |
|
Median OS (range, months) |
21.30-69.60 |
24.90-69.60 |
21.30-23.90 |
|
Median PFS (range, months) |
3.60-14.10 |
11.00-14.10 |
3.60-10.90 |
|
Median follow-up time (range, months) |
6.00-55.20 |
12.00-55.20 |
6.00-21.00 |
|
SD: Standard deviation, N/A: Not available, MWA: Microwave ablation, CT: Computed tomography,MHz: Megahertz, Max: Maximum, W: Watt, cm: centimeter, mm: millimeter, G: Gauge, min: Minute, PTX: Pneumothorax, PE: Pleural effusion, OS: Overall survival, PFS: Progression-free survival. |
|||
MWA and Chemotherapy Characteristics
MWA was used as a standalone treatment in 592 cases (63.80%) and combination with chemotherapy in 336 cases (36.20%). Computed tomography scan was consistently utilized for imaging guidance in all cases. The MWA frequency was predominantly set at 2450 MHz in 705 cases (75.97%). The most commonly used antenna specifications included a maximum length of 180 mm in 690 cases (74.35%) and a gauge of 20 in 390 cases (42.03%). For cases where data were available, the active tip length of the antenna was primarily 15 mm (15.41%). MWA procedures were frequently performed under local anesthesia in 657 cases (70.80%). The mean power used was 71.76 ± 21.86 watts, with 80 watts being the most common setting in 279 cases (30.06%). The average duration for maximum ablation was 14.38 ± 7.50 minutes. Regarding the ablation zone, a maximum extension of 10 mm beyond the tumor margin was used in 481 cases (51.83%) and 5 mm in 187 cases (20.15%), while the margin was unknown in the remaining cases (28.02%). The chemotherapy regimen included pemetrexed (41.37%), docetaxel (12.20%), paclitaxel (5.06%), gemcitabine (8.93%), tigio (0.89%), cisplatin (11.90%), carboplatin (5.65%), and nedaplatin (26.49%) (Table 3).
Safety and Efficacy
Complications were more prevalent in patients who underwent MWA alone (59.29%) compared to those who received MWA combined with chemotherapy (32.74%). The most frequent complication was pneumothorax, occurring in 176 cases (18.97%), with 140 cases (23.65%) in the MWA alone group and 36 cases (10.71%) in the combination group. Among the complications, only infection was higher in the MWA combined with the chemotherapy group (4.76% vs. 1.86%). Patients with available data and treated with MWA alone experienced higher local progression (26% vs. 18.5%), distant recurrence (51.5% vs. 38.5%), and both local and distant recurrence (10.8% vs. 2.6%). Reported complete response was 88.6% among cases that underwent MWA alone. While it was 78.0% in those who underwent combined MWA and chemotherapy. The median OS was higher in cases that underwent MWA alone (24.9 to 69.6 months vs. 21.3 to 23.90 months). Despite that, a significant number of studies across both treatment groups did not provide comprehensive recurrence data. Specifically, ten studies, encompassing 572 cases (61.63%), did not report on local recurrence, while eleven studies, involving 442 cases (47.63%), lacked data on distant recurrence. Additionally, thirteen studies, including 778 cases (83.84%), failed to report on both local and distant recurrence. Furthermore, ten studies covering 585 cases (63.04%) omitted information on the complete response, and eleven studies involving 707 cases (76.18%) did not document partial response (Table 3).
Discussion
It has been indicated that NSCLC tumors develop through progressive pathological changes and exhibit a few unique molecular signatures of genomic alterations. The alterations typically occur in the cells lining the airways, which are predominantly exposed to harmful chemicals such as tobacco smoke carcinogens and environmental pollutants like asbestos, nickel, and arsenic. As these precancerous cells progress through various stages of tumorigenesis, they undergo changes, including hyperplasia, squamous metaplasia, squamous dysplasia, and eventually carcinoma in situ [3].
Xu et al. conducted a cohort study involving 319 patients undergoing MWA for NSCLC management, reported a mean age of 68.0 ± 10.6 years, with a male predominance (61.4%) [25]. Similarly, the present study found a mean age of 69.52 ± 9.07 years, with a similar male representation (62.50%) in the MWA alone group. In an RCT by Wei et al., which examined the use of MWA combined with chemotherapy for NSCLC management, the mean age of patients was 59 years, with a male predominance (65%) [22]. However, the mean age of cases who underwent MWA combined with chemotherapy in the present study was 62.28 ± 6.96 years, with a male ratio of 63.10%. The group of MWA alone had a smaller mean tumor size of 2.93 ± 0.72 cm compared to a study by Yao et al., which reported a mean tumor size of 3.01 ± 1.11 cm [19]. Conversely, the group of MWA combined with chemotherapy had a larger mean tumor size of 3.86 ± 0.24 cm, which was greater than the 3.6 cm reported in another study [22].
Primarily, there are three types of NSCLC: adenocarcinoma, which accounts for 40% of cases; squamous cell carcinoma (25-30%) and large cell carcinoma (5-10%) [26]. In this systematic review, adenocarcinoma was also the predominant subtype, but at a higher percentage of 65.52%. Squamous cell carcinoma was found in 19.50%, consistent with expected patterns, while large cell carcinoma was identified in only 1.29%, significantly lower than that reported. Lv et al. reported that, among 118 cases, the most common tumor locations were the upper and middle lobes of the lung, observed in 73 patients (61.86%), while 45 patients (38.14%) had the tumor in the lower lobe [12]. In the present study, in 258 cases (27.80%), the tumors were located in the upper and middle lobes, while in 143 cases (15.41%), the lower lobe was involved. However, a substantial proportion of the studies (56.79%) had missing data regarding tumor location.
MWA has emerged as a promising option for treating NSCLC, particularly in cases where surgery is not feasible. Since its introduction in 2002, MWA has gained recognition as a viable alternative for early-stage NSCLC [8]. Wu et al. reported that percutaneous image-guided ablation, including MWA, offers comparable OS rates to stereotactic ablative radiotherapy for inoperable early-stage primary lung cancer. Additionally, propensity-score-weighted analyses have demonstrated that MWA achieves OS and disease-free survival rates similar to surgery [8]. Li et al. highlighted the advantages of MWA's minimally invasive nature, making it a favorable choice for preserving pulmonary function. This treatment is particularly suitable for stage I NSCLC patients who are medically inoperable due to high-risk conditions or who prefer not to undergo surgery [14]. Shan et al. also emphasized MWA's efficacy in effectively destroying tumor cells, with some patients achieving outcomes comparable to surgery. This reduction in tumor burden improves tumor-free survival and enhances patients' quality of life and mental well-being [7]. Furthermore, Huang et al. underscored the potential of MWA not only for inoperable early-stage NSCLC but also as a palliative option for advanced NSCLC when combined with systemic chemotherapy. This combination could significantly extend both progression-free survival (PFS) and OS [27]. These studies highlight MWA as an effective and versatile treatment option across different stages of NSCLC. In this systematic review, 52.87% of the patients in the MWA alone group had early-stage NSCLC, while 13.85% were diagnosed with advanced disease. In contrast, the disease in all patients (100%) in MWA combined with the chemotherapy group was advanced.
A study reported a maximum ablation time of 8 minutes [15], while another one utilized a maximum ablation time of 20 minutes [9]. In contrast, this systematic review showed the mean maximum ablation time of 11.86 ± 4.80 minutes in the MWA alone group. This indicates that the ablation times reported in individual studies fell within the broader range observed in the systematic review, exhibiting some variation but generally consistent with the overall average. Wei et al. conducted an RCT on patients who received MWA combined with chemotherapy, using a maximum ablation time of 8 minutes [22]. Conversely, another study reported a maximum ablation time of 33 minutes [17]. In the present study, the mean maximum ablation time for cases who underwent MWA combined with chemotherapy was 18.40 ± 9.76 minutes. This indicates a notable increase in ablation time when MWA is combined with chemotherapy.
Regarding safety, Xu et al., among 391 cases of NSCLC underwent MWA, reported a complication rate of 60.50%, with pneumothorax being the most common complication (49.22%), followed by emphysema (42.49%) [25]. Similarly, the complication rate among cases who underwent only MWA in the current study was 59.29%, higher than the other group (32.74%), with pneumothorax being the most common complication (23.65%), followed by pleural effusion (9.12%). A study conducted in 2019 reported a local progression rate of 26.79% after MWA treatment [16]. In contrast, another study found a significantly lower incidence of local progression of 8.82% [28]. In this study, among the cases of the MWA alone group with available data, the incidence of local progression was 25.97%. In patients treated with MWA combined with chemotherapy, one study reported a local progression rate of 18.24% [22]. Another study found a similar local progression rate of 17.95% [24]. In this study, the local progression rate in combined regimens was 18.45% for available case data, closely aligning with the reported incidence rates [11,20]. In the current study, local recurrence occurred in 87 cases out of 356 (24.44%) with available data in the MWA alone group. Meanwhile, Wei et al. A local recurrence rate of 6.33% was reported, and Yang et al. observed a higher rate of 27.66% [20,29]. The reviewed studies on MWA combined with chemotherapy did not provide any data on local recurrence, highlighting a significant gap in the reported outcomes for this group.
Within the reviewed cases with available data who underwent MWA alone, the treatment resulted in a complete response rate of 88.6% (70 out of 79 cases) and a partial response rate of 15.2 % (5 out of 33 cases). Wei Z et al. reported a slightly higher complete response rate of 91.14% with a lower partial response rate of 8.86% [29]. Another study found a complete response rate of 75% and a partial response rate of 25% with the same treatment modality [28]. In the group of MWA combined with chemotherapy of this study, the complete and partial response rates were 78.0% and 41.0%, respectively. However, the high percentage of cases with unknown data regarding response rate should not be overlooked. Shan et al. reported a complete response rate of 23.53% and a partial response rate of 47.06% [7]. In contrast, Wei et al. observed a higher complete response rate of 84.78% and a lower partial response rate of 15.22% [23].
Xu et al. reported a median OS of 17.0 ± 10.9 months and a median PFS of 13.0 ± 10.5 months in patients treated with MWA [25]. Additionally, another study documented a median OS of 20 months [30]. The studies included in the group of MWA alone of this systematic review reported a broader range of outcomes, with median OS varying from 24.90 to 69.60 months and median PFS ranging from 11.00 to 14.10 months. The median OS for that group was generally higher than that reported by Xu et al. and Pusceddu et al. [25,30]. In addition, a study by Huang et al. reported a median OS of 18.8 months and a median PFS of 8.1 months for patients treated with MWA combined with chemotherapy [27]. The patients with the combined regimen in the present study showed slightly higher median OS values, ranging from 21.30 to 23.90 months, but a broader range of median PFS, from 3.60 to 10.90 months.
This study was constrained by the significant absence of critical outcome data in many studies, including local and distant recurrence rates, treatment responses, and survival rates. Specifically, the lack of local and distant recurrence data in 61.63% and 47.63% of cases, respectively, impairs a comprehensive assessment of the efficacy of MWA alone or in combination with chemotherapy.
Conclusion
MWA combined with chemotherapy may represent a more effective option, with a slightly similar treatment response, reducing the risk of recurrence and minimizing complications. However, the influence of the tumor stage on outcomes may not be excluded.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: MGHH, BAA, AMA, and YMM were responsible for the manuscript's data collection, analysis, and final approval. FHK and AMS were significant contributors to the conception of the study, as well as to the literature search for related studies. RMA, SHT, AMS, LRAP, and AJQ were involved in the literature review, the design of the study, and the critical revision of the manuscript. SHM, HOA, HKA, and FA were involved in the literature review, the writing of the manuscript, and the design of the study and interpretation. BAA and FHK confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
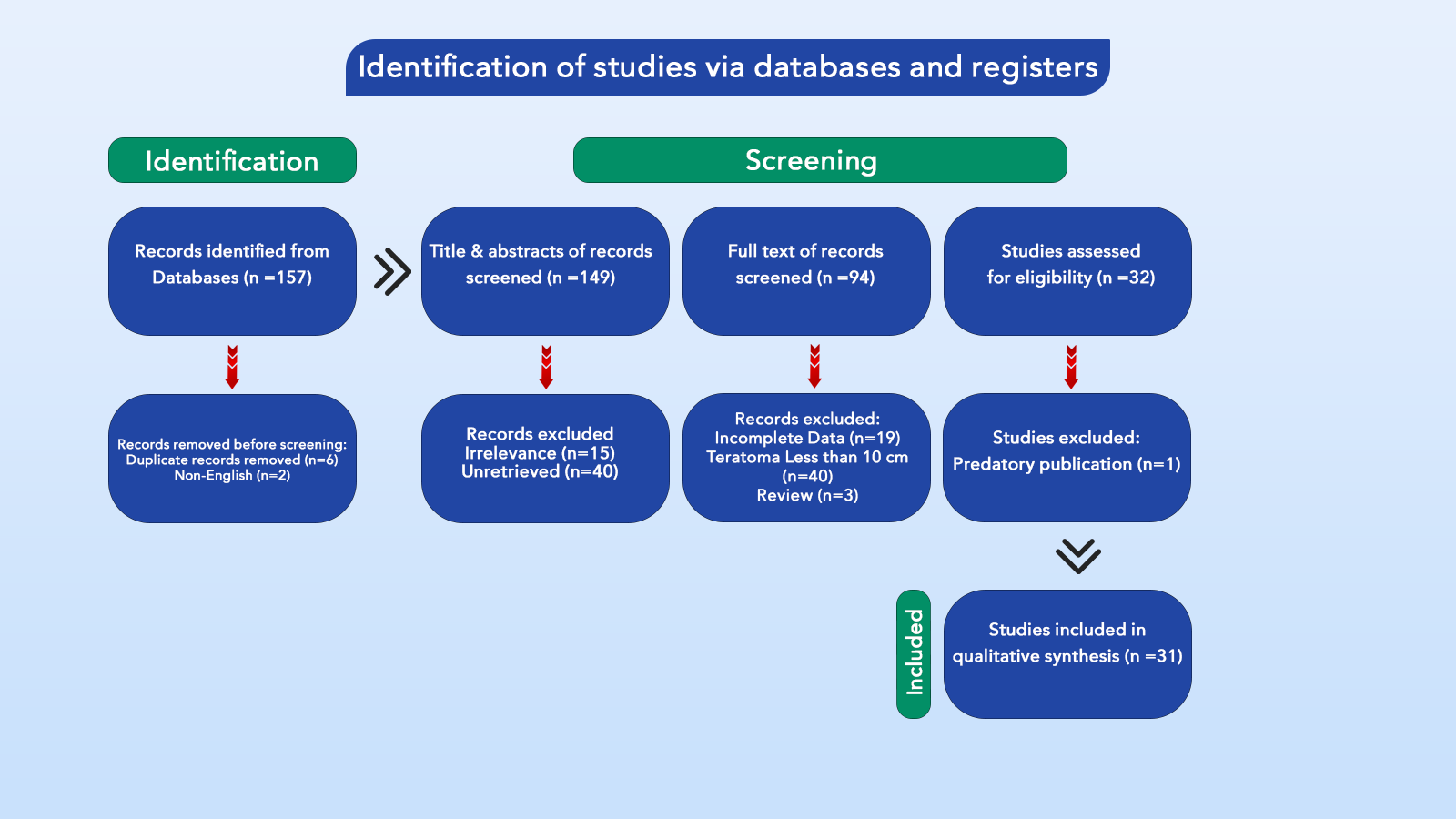
Giant Sacrococcygeal Teratoma in Infant: Systematic Review
Gauri Shankar Shah, Satoshi Ieiri, Wirya N. Sabir, Bilal A. Mohammed, Shwan Fakhrulddin...
Abstract
Introduction
Sacrococcygeal teratoma (SCT) is a rare embryonal tumor that occurs in the sacrococcygeal region, with an incidence of about 1 in 35,000 to 40,000 live births. This study presents a systematic review of giant SCT greater than 10 cm.
Methods
A systematic review of published studies regarding giant SCT in infants was conducted. The studies included met the following criteria: 1) the teratoma was situated in the sacrococcygeal region; 2) all case reports involved infants with a teratoma larger than 10 cm, and 3) the size of the teratoma was verified to exceed 10 cm through diagnostic methods.
Results
The current study included 31 studies that met the inclusion criteria. The studies included patients aged 6.1 to 9.3 months, with a median age of 7.6 months, predominantly female (72.7%). Diagnoses were primarily made in the first and second trimesters (39%) or after birth (33.3%), with cesarean delivery being the most common method (66.7%). Tumors weighed between 1.5 and 5 kg, with an average diameter of 15.6 cm. Surgical resection was performed in 93.9% of cases. The most common complication was respiratory failure (30.3%), and histopathology revealed that 39.4% of tumors were immature teratomas, while 33.3% were mature teratomas. The overall survival rate was 66.7%, with 18.2% of survivors experiencing tumor recurrence. Most complications occurred in the second trimester; however, no significant associations were found concerning the timing of diagnosis. Additionally, tumor size did not significantly impact outcomes.
Conclusion
Routine ultrasound and MRI are essential for the antenatal diagnosis of SCT. Due to the high risk of morbidity with larger tumors, cesarean delivery is advised for tumors over 10 cm. Coccygectomy is the most effective approach to prevent recurrence, highlighting the importance of timely surgical intervention and ongoing follow-up.
Introduction
Sacrococcygeal teratoma (SCT) is an uncommon embryonal tumor form in the sacrococcygeal region. It affects around one among every 35,000 to 40,000 live births [1]. The condition is significantly more common in females, with a female-to-male ratio of 3:1 to 4:1. Teratomas consist of tissues originating from all three germ layers: ectoderm, endoderm, and mesoderm [2,3]. Although their exact embryonic origin remains uncertain, SCTs are believed to arise early in gestation from totipotent cells in Hensen’s node, a remnant of the primitive streak in the coccygeal region [2]. SCTs can vary significantly in size, with some growing large enough to cause noticeable anatomical changes, such as the anterior displacement of the anus and resulting in clinical symptoms like anal displacement, tightening of the anal canal, and constipation, often due to tumor compression of the bladder or rectum [1,3]. Obstetric ultrasound during the second trimester is a crucial tool for making antenatal diagnoses of tumors, helping to prevent perinatal and neonatal complications such as fetal hydrops, tumor rupture, placentomegaly, and high-output cardiac failure, all of which are linked to a higher risk of mortality [4,5]. In 1973, according to the American Academy of Pediatrics Surgical Section (AAPSS), Altman classified SCTs into four types based on the tumor's intrapelvic and intra-abdominal extension and external components. SCTs observed at birth are typically classified as Altman Type I or II, with Type III being rare, while Type IV is usually identified later in life [2]. The management of SCT involves surgical excision, and the likelihood of recurrence after complete removal is minimal [5], leading to a favorable prognosis. However, long-term follow-up is essential to monitor for recurrence [3].
While there have been reports of large SCTs, to the best of our knowledge, based on an extensive literature review and previous case reports, no systematic reviews specifically address SCTs greater than 10 cm in infants. All included references were confirmed for eligibility [6].
Methods
Study design
The present systematic review adhered to the preferred reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and search strategy
A comprehensive review of all published studies on SCT was conducted by searching databases such as Google Scholar, PubMed/MEDLINE, Cochrane Library, ScienceDirect, CINAHL, Web of Science, and EMBASE. The search used the following keywords: (sacrococcygeal) AND (teratoma OR tumor) in combination with (antenatal OR neonate OR infancy OR infant OR child OR children OR newborn OR pediatric).
Eligibility criteria
The non-English studies and those unrelated to humans were excluded before or during the initial screening. Studies on SCT were included if they met the following criteria: 1) the teratoma was located in the sacrococcygeal region; 2) all case reports involved infants with a teratoma larger than 10 cm, and 3) the teratoma size was confirmed to be over 10 cm through diagnostic methods. Studies published in predatory journals (inappropriately peer-reviewed) and those not meeting the inclusion criteria were excluded.
Study selection and data extraction
The titles and abstracts of the identified studies were initially screened. This was followed by a comprehensive review of the full text to assess eligibility. Data points collected from the included studies included the year and country of the study, patient age and gender, age at delivery, trimester of detection, postnatal diagnosis, mode of delivery, tumor size and weight, imaging technique, complications, and recurrence rates.
Statistical analysis
The data were first utilized in a qualitative synthesis, followed by quantitative re-analysis using the Chi-square test and Fisher's exact test for categorical variables and the independent sample t-test for numerical data, conducted with SPSS software version 25.0. A significance level of 0.05 was not established.
Results
Study selection and characteristics
A systematic search initially identified 157 articles. Before screening, eight were excluded due to duplication and non-English language. During the initial title and abstract screening, 55 studies were excluded as they did not meet the inclusion criteria. Subsequently, 94 studies underwent full-text screening, and 32 were further assessed for eligibility. Ultimately, 31 studies [1-4, 7-33] were compatible with the inclusion criteria and included in the final analysis (Figure 1). All the studies included were case reports (Table 1).
|
Author [reference] |
Year |
Country |
Gender |
Age of delivery (days) |
Diagnosis time |
Mode of delivery |
Weight (kg) |
Size of tumor (cm) |
Altman classification |
Complication |
Histopathology |
Survival |
Recurrence |
|
Chamberlin et al. [7] |
N/A |
USA |
N/A |
224 |
1st trimester |
CS |
3.7 |
13.9 |
N/A |
Near cardiac arrest & hypoxia |
N/A |
Yes |
N/A |
|
Sabir et al. [1] |
2023 |
Iraq |
F |
266 |
2nd trimester |
CS |
5.0 |
17.5 |
1 |
None |
Immature teratoma |
Yes |
No |
|
Dey et al. [8] |
2023 |
USA |
F |
206 |
Prenatal (Uknown trimester) |
CS |
2.4 |
11.6 |
3 |
Poor respiratory effort |
Immature teratoma |
Yes |
No |
|
Abou-Bekr et al. [4] |
2022 |
Algeria |
F |
N/A |
Postnatal |
CS |
N/A |
10 |
1 |
None |
Mature teratoma |
Yes |
N/A |
|
Koc et al. [9] |
2022 |
Turkey |
F |
209 |
N/A |
N/A |
2.2 |
10 |
1 |
None |
Teratoma (unknown type) |
Yes |
N/A |
|
Meshram et al. [2] |
2021 |
India |
M |
259 |
2nd trimester |
CS |
2.2 |
24.5 |
2 |
Polyhydramnios |
Mature teratoma |
No |
N/A |
|
Zlatan et al. [10] |
2021 |
Bosnia and Herzegovina |
M |
238 |
2nd trimester |
CS |
2.3 |
15.5 |
2 |
Cardiac arrest |
Immature teratoma |
Yes |
N/A |
|
Guitart et al. [11] |
2020 |
Spain |
N/A |
245 |
2nd trimester |
CS |
4.0 |
18 |
1 |
Premature rupture of the membranes & abnormal cardiotocography |
Immature teratoma |
Yes |
N/A |
|
Savitri et al. [12] |
2019 |
Indonesia |
F |
266 |
2nd trimester |
CS |
4.2 |
11.2 |
1 |
Resuscitation needed |
Teratoma (unknown type) |
Yes |
No |
|
Singhal et al. [13] |
2018 |
India |
F |
196 |
Postnatal |
NVD |
3.6 |
16 |
N/A |
Infection |
Mature teratoma |
N/A |
N/A |
|
Konoplitskyi et.al [14] |
2018 |
Ukraine |
F |
280 |
3rd trimester |
CS |
4.3 |
14.9 |
1 |
N/A |
Immature teratoma |
Yes |
Yes |
|
Sop Lee et al. [15] |
2017 |
Korea |
M |
199 |
2nd trimester |
CS |
2.9 |
12.3 |
3 |
Poor respiratory distress |
Immature teratoma |
Yes |
No |
|
Bechtel et.al [16] |
2014 |
USA |
N/A |
226 |
Prenatal (Uknown trimester) |
CS |
3.7 |
13.9 |
2 |
Lower limb flaccid paralysis, scoliosis |
Immature teratoma |
Yes |
Yes |
|
Mondal et al. [17] |
2014 |
India |
F |
245 |
Postnatal |
NVD |
3.5 |
16.7 |
1 |
Bleeding |
N/A |
No |
N/A |
|
Mbumba et al. [18] |
2010 |
France |
F |
266 |
1st trimester |
CS |
N/A |
16 |
N/A |
None |
Mature teratoma |
N/A |
N/A |
|
Roka et al. [3] |
2010 |
Nepal |
F |
N/A |
Postnatal |
N/A |
N/A |
22.4 |
N/A |
None |
Mature and yolk sac components (Schiller-Duval bodies) |
Yes |
N/A |
|
Lahdes-Vasama et al. [19] |
2010 |
Finland |
F |
210 |
2nd trimester |
CS |
3.4 |
16.9 |
2 |
Bleeding |
Immature teratoma with a malignant component in a small area |
Yes |
No |
|
Abraham et al. [20] |
2010 |
USA |
F |
238 |
2nd trimester |
CS |
N/A |
18.9 |
3 |
Premature rupture of membranes & poor respiratory effort |
N/A |
Yes |
N/A |
|
Den Otter et al. [21] |
2007 |
Netherlands |
F |
192 |
2nd trimester |
CS |
2.2 |
11 |
2 |
Respiratory failure |
Mixed teratoma |
Yes |
Yes |
|
Howman-Giles et al. [22] |
2007 |
Australia |
F |
N/A |
Postnatal |
N/A |
N/A |
16 |
N/A |
Left iliac lymph node enlargement, colostomy needed |
Immature teratoma |
No |
Yes |
|
Hosono et al. [23] |
2004 |
Japan |
F |
252 |
3rd trimester |
CS |
4.0 |
13.1 |
1 |
Poor respiratory efforts |
Immature teratoma |
Yes |
No |
|
Ribeiro et al. [24] |
1999 |
France |
F |
266 |
Postnatal |
CS |
2.7 |
11.4 |
1 |
None |
Mature teratoma |
Yes |
No |
|
Jona et al. [25] |
1999 |
USA |
F |
189 |
Prenatal (Uknown trimester) |
CS |
1.7 |
26 |
N/A |
N/A |
Teratoma (unknown type) |
No |
N/A |
|
Johnston [26] |
1998 |
USA |
N/A |
245 |
N/A |
N/A |
3.9 |
12.2 |
N/A |
N/A |
Mature elements, except for immature neuroectodermal tissue. |
Yes |
N/A |
|
Robertson et al. [27] |
1995 |
USA |
F |
185 |
2nd trimester |
CS |
1.8 |
N/A |
2 |
Severe respiratory distress syndrome |
Mature teratoma |
Yes |
No |
|
Lnoue et al. [28] |
1994 |
Japan |
M |
210 |
2nd trimester |
CS |
3.8 |
10 |
N/A |
Bleeding and circulatory failure |
Immature teratoma |
Yes |
N/A |
|
Nakayama et al. [29] |
1991 |
USA |
F |
231 |
3rd trimester |
CS |
4.3 |
13.9 |
2 |
Respiratory insufficiency & renal failure |
Immature teratoma |
N/A |
N/A |
|
1991 |
USA |
F |
210 |
Postnatal |
CS |
3.0 |
15 |
3 |
Respiratory distress |
Immature teratoma |
N/A |
N/A |
|
|
Worsham et al. [30] |
1975 |
USA |
F |
189 |
Postnatal |
NVD |
1.9 |
13.1 |
N/A |
No respiratory efforts |
N/A |
No |
N/A |
|
Williams et al. [31] |
1970 |
Nigeria |
F |
266 |
Postnatal |
N/A |
N/A |
30 |
N/A |
Metastasis |
Teratoma (unknown type) |
Yes |
Yes |
|
Schiffer et al. [32] |
1956 |
USA |
F |
196 |
3rd trimester |
NVD |
1.5 |
20 |
N/A |
Difficult birth |
N/A |
N/A |
N/A |
|
1956 |
USA |
N/A |
N/A |
Postnatal |
NVD |
N/A |
15 |
N/A |
Difficult birth |
N/A |
N/A |
N/A |
|
|
Walker et al. [33] |
1950 |
USA |
F |
224 |
Postnatal |
NVD |
2.7 |
12.5 |
N/A |
N/A |
Teratoma (unknown type) |
Yes |
N/A |
| N/A: non-available, F: female, M: male, CS: cesarean section, NVD: normal vaginal delivery | |||||||||||||
Patient characteristics, diagnosis, management and outcome
The included studies characterized patients with delivery ages ranging from 6.1 to 9.3 months, with a median age of 7.6 months. Most patients were female (72.7%), while 12.1% were male, and in 15.2% of cases, gender was unspecified. Diagnoses were predominantly made during the first and second trimesters (39%) or after birth (33.3%), with cesarean section as the most common delivery mode (66.7%). Tumor weights ranged from 1.5 to 5 kg, with an average diameter of 15.6 cm. Surgical resection was the primary treatment, performed in 93.9% of cases, although two patients died before surgery could be undertaken. Complications were frequent, with respiratory failure as the most common (30.3%). Histopathological analysis indicated that 39.4% of tumors were immature teratomas and 33.3% were mature teratomas. The overall survival rate was 66.7%, and 18.2% of surviving patients experienced tumor recurrence (Table 2).
|
Variables |
Frequency / Percentage |
|
Patient demographics |
|
|
Age at delivery time, range (median, mean ± SD), month |
6.1 – 9.3 (7.6, 7.6 ± 0.97) |
|
Gender Male Female N/A |
4 (12.1%) 24 (72.7%) 5 (15.2%) |
|
Diagnosis time In the first trimester In the second trimester In the third trimester Diagnosed prenatally with unknown trimester Postnatal Unknown |
2 (6.1%) 11 (33.3%) 4 (12.1%) 3 (9.1%) 11 (33.3%) 2 (6.1%) |
|
Mode of delivery Cesarean section Normal vaginal delivery Unknown |
22 (66.7%) 6 (18.2%) 5 (15.1%) |
|
Weight of the tumor (kg), range (mean ± SD) |
1.5 – 5 (3.1 ± 0.96) |
|
Tumor size (cm), range (mean ± SD) |
10 – 30 (15.6 ± 4.77) |
|
Group of tumors (Altman classification) Group 1 Group 2 Group 3 Unknown |
9 (27.3%) 7 (21.2%) 4 (12.1%) 13 (39.4%) |
|
Management Surgical resection Patient died before surgery |
31 (93.9%) 2 (6.1%) |
|
Complications Yes No Unknown |
21 (63.6%) 10 (30.3%) 2 (6.1%) |
|
Common perioperative complications* Respiratory failure Bleeding Cardiac failure/problem Infection |
10 (30.3%) 3 (9.1%) 3 (9.1%) 2 (6.1%) |
|
Histopathology Immature teratoma Mature teratoma Mixed teratoma Unknown |
13 (39.4%) 11 (33.3%) 3 (9.1%) 6 (18.2%) |
|
Survival Yes No Unknown |
22 (66.7%) 5 (15.1%) 6 (18.2%) |
|
Recurrence among survived cases Yes No Unknown |
4 (18.2%) 8 (36.4%) 10 (45.4%) |
|
*Other complications may have been reported N/A: non-available, SD: standard deviation |
|
Complications and recurrence
The comparative analysis of complications assessed the trimester of diagnosis, timing of diagnosis, and recurrence rates. Most complications occurred in the second trimester (10 cases), with fewer cases reported in the first and third trimesters; however, the p-value of 0.34 indicates no significant difference across trimesters. Diagnostic timing was categorized into prenatal and postnatal periods. Complications were more common in the 20 cases diagnosed prenatally (16 cases) than in postnatal diagnoses, but Fisher’s exact test yielded a p-value of 0.10, suggesting no statistically significant association. For recurrence, complication rates were similar in both recurrence and non-recurrence groups, with a p-value of 0.55, supporting the lack of a substantial correlation. Overall findings reveal no strong statistical relationships between complications and diagnostic timing, trimester of diagnosis, or recurrence (Table 3). The influence of tumor size and weight on complications, recurrence, and survival is demonstrated. The mean tumor size was slightly larger in cases with complications (15.02 ± 3.49 cm) compared to those without complications (14.29 ± 3.87 cm), but the difference was not statistically significant (p = 0.6). Recurrence was associated with larger tumors (17.16 ± 7.41 cm) relative to non-recurrent cases (13.42 ± 2.66 cm), although this difference was not significant (p = 0.14). Similarly, non-survivors exhibited a higher mean tumor size (19.25 ± 5.65 cm) compared to survivors (14.6 ± 4.84 cm), but the association was not statistically meaningful (p = 0.41). For tumor weight, no significant variations were found across outcomes. The mean tumor weight in cases with complications was 3.03 ± 0.88 kg versus 3.47 ± 1.09 kg in those without (p = 0.39). Likewise, tumor weight did not show significant differences for recurrence (p = 0.92) or survival (p = 0.35). The findings indicate that neither tumor size nor weight significantly impacted complications, recurrence, or survival (Table 4).
|
Variables |
Complication |
Total |
P-value |
|
|
Yes |
No |
|||
|
Trimester at diagnosis First Second Third |
1 10 3 |
1 1 1 |
2 11 4 |
0.34 |
|
Diagnosis time Prenatal Postnatal |
16 5 |
4 6 |
20 11 |
0.10* |
|
Recurrence Yes |
2 6 |
2 2 |
4 8 |
0.55* |
|
CS; cesarean section, NVD; normal vaginal delivery *Fisher's exact test |
||||
|
Tumor characteristics |
Complication |
P-value |
Recurrence |
P-value |
Survival |
P-value | |||
|
Yes |
No |
Yes |
No |
Yes |
No |
||||
|
Tumor size (mean ±SD) |
15.02 ± 3.49 |
14.29 ± 3.87 |
0.6 |
17.16 ± 7.41 |
13.42 ± 2.66 |
0.14 |
14.6 ± 4.84 |
19.25 ± 5.65 |
0.41 |
|
Tumor weight (mean ±SD) |
3.03 ± 0.88 |
3.47 ± 1.09 |
0.39 |
3.40 ± 1.08 |
3.31 ± 1.04 |
0.92 |
3.25 ± 0.91 |
2.34 ± 0.80 |
0.35 |
| SD; standard deviation | |||||||||
Discussion
SCTs are rare embryonal tumors that develop in the sacrococcygeal region of newborns, the most common site for germ cell tumors. While SCTs occur more frequently in females—with 72.7% of cases in this systematic review involving female patients, they tend to show a higher malignancy rate in males [16]. Most of these tumors are histologically benign and can be classified into three main types: mature teratomas, which consist of fully differentiated tissues such as bone, teeth, and hair; immature teratomas, which contain embryonal elements or partially differentiated structures that pose a significant risk of malignancy; and malignant teratomas, which include one or more malignant germ cell tumors such as yolk sac tumors, choriocarcinomas, and embryonal carcinomas [5,21].
SCTs are classified into four types based on the location of internal and external tumors, as defined by the AAPSS. Type I involves an externally visible mass; Type II is characterized by an external mass with a significant intrapelvic component; Type III includes both external and pelvic masses; and Type IV is entirely internal [17]. Prenatal ultrasound (U/S) can effectively identify Types I and II, while Type IV poses a higher risk of malignancy due to its internal location. Routine U/S is typically performed during the second trimester for antenatal diagnosis, though detection is possible as early as the first trimester. In accordance with this data, more than 60 percent of cases in this systematic review are diagnosed prenatally. However, many pregnant women skip first-trimester U/S screenings, making early detection of masses more challenging. In some cases, the mass is large enough to be detected earlier [1].
Magnetic resonance (MR) imaging complements ultrasound by providing a more detailed characterization of the mass’s tissue components [16]. It is particularly useful for evaluating the full extent of large lesions. MR imaging can accurately determine the tumor’s reach and the pressure it exerts on surrounding organs. It also aids in distinguishing this condition from common differential diagnoses, such as distal neural tube defects, including myelocystocele or myelomeningocele [5].
Additionally, elevated levels of tumor markers, such as alpha-fetoprotein (AFP), are used to assess the likelihood of malignancy. High AFP levels may indicate a malignant tumor; however, in newborns, AFP levels are naturally elevated and typically return to normal by around nine months of age, leading to potential misdiagnosis of the condition as malignant [1].
Perinatal morbidity and mortality rates are notably elevated in fetuses with SCT, primarily due to the high frequency of preterm birth. The most severe perinatal complications include premature labor, malignant tumor infiltration, hemorrhage or rupture of the tumor, amniotic fluid obstruction, and heart failure [5]. Factors that increase the risk of complications include rapid tumor growth exceeding 150 cm³ per week, tumor size greater than 10 cm, highly vascularized solid tumors, polyhydramnios, and cardiac decompensation [4,21].
Cesarean delivery is recommended for mothers carrying fetuses with SCTs larger than 10 cm in diameter, especially when the tumors are highly vascularized or exceed 5 cm. This approach minimizes the risk of tumor rupture and hemorrhage [2]. This systematic review indicates that cesarean section is performed in more than two-thirds of such cases. The incision is typically made in the lower uterine segment, as the uterus often becomes significantly enlarged due to the tumor's size [21].
Timely surgical intervention for SCTs is crucial, particularly when the tumor exceeds 10 cm, as delays can increase the risk of malignancy and recurrence. Surgery is the primary treatment, with over 93% of cases requiring surgical management. Current expert recommendations indicate that coccygectomy is the most effective approach for preventing the recurrence of benign teratomas, with minimal risk to the tumor cyst wall [2,4]. Ideally, this procedure should be performed within one week and not delayed beyond that period. During surgery, careful fetal monitoring is essential to prevent operative mortality, often caused by severe hemorrhage or cardiac arrest, frequently linked to hyperkalemia [4,10]. Additionally, ensuring the availability of cross-matched blood in the operating room is critical [4].
When the coccyx is not removed, the likelihood of cancer recurrence can be as high as 37%. Even when patients have the entire coccyx removed, there is still a chance of the cancer returning, ranging from 11% to 22% [2]. This aligns with the 18% recurrence rate mentioned in this review. Although recurrence is uncommon, several factors could contribute to it, such as incomplete removal of the tumor, failure to completely remove the coccyx along with the tumor, tumor spillage or rupture, and undetected malignant components within the tumor [12].
Although survival outcomes for SCTs are generally positive, the mortality rate for tumors larger than 10 cm is reported to be around 18%, which is nearly similar to the 15% rate mentioned in this review. SCTs can potentially recur years after treatment, highlighting the importance of continuous monitoring into adulthood. Regular follow-up appointments are recommended every 3 to 6 months, including physical examinations, such as rectal exams, diagnostic imaging, and alpha-fetoprotein (AFP) testing, for at least three years [1,16].
Conclusion
Routine ultrasound and magnetic resonance imaging play critical roles in the antenatal diagnosis and characterization of SCTs. Given the elevated perinatal morbidity and mortality associated with SCTs, especially due to preterm birth, cesarean delivery is advised for tumors larger than 10 cm to reduce risks of rupture and bleeding. Furthermore, coccygectomy is highlighted as the most effective strategy for preventing the recurrence of benign teratomas, underscoring the importance of timely and thorough intervention in managing these cases.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: PAN, and BAA were responsible for the manuscript's data collection, analysis, and final approval. GSS, WNS and SI were significant contributors to the conception of the study, as well as to the literature search for related studies. BAM, SFA, and SMA were involved in the literature review, the design of the study, and the critical revision of the manuscript. SMA and PAN were involved in the literature review, the writing of the manuscript, and the design of the study and interpretation. WNS and SMA confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
Case Reports
Phrenic Nerve Block for Management of Post-Thoracic Outlet Decompression Cough: A Case Report and Literature Review
Fahmi H. Kakamad, Soran H. Tahir, Nsren S. Sabr, Rezheen J. Rashid, Saywan Kakarash Asaad,...
Abstract
Introduction
Thoracic outlet syndrome is a group of disorders arising from compressive forces on the neurovascular bundle in that region due to different etiologies. This study aims to present a case with an intractable cough as a complication of the surgery and its management.
Case presentation
A 41-year-old woman had a 15-year history of bilateral upper limb pain and numbness. The pain worsened over the last two months. Tests and imaging confirmed thoracic outlet syndrome. Physiotherapy had no effect. She underwent thoracic outlet decompression (TOD) under general anesthesia via a supraclavicular incision. Post-surgery, she developed uncontrollable cough that didn't respond to medication. Two days after the operation, a bupivacaine (5%) injection near the phrenic nerve resolved the cough.
Literature review
The literature review identified several cases of TOD complications, including right phrenic nerve paralysis resolving in 3-6 months, unilateral right diaphragm dysfunction, and lung herniation successfully treated with thoracoscopic reduction. These cases underscore the diverse presentations and outcomes of TOD complications, highlighting the importance of early diagnosis and tailored management strategies.
Conclusion
A possible complication of surgical decompression for thoracic outlet syndrome is an intractable cough that can be relieved by the injection of local anesthesia into the area of the phrenic nerve.
Introduction
Thoracic outlet syndrome (TOS) encompasses a range of disorders that lead to the compression of the neurovascular bundle in the thoracic outlet [1,2]. It is commonly classified into three types: neurogenic TOS (nTOS), venous TOS (vTOS), and arterial TOS (aTOS) [3]. nTOS is the predominant type, representing more than 90% of the cases. TOS is more commonly seen in females and individuals who have poor muscle development, poor posture, or both. Determining the precise prevalence of TOS is challenging due to the non-specific nature of its symptoms. The estimated incidence of TOS ranges widely, with approximately 3 to 80 cases per 1000 people. TOS typically occurs in three distinct spaces: the scalene triangle, costoclavicular space, and subcoracoid space. This condition can arise due to congenital, acquired, or traumatic factors. Secondary causes include clavicular fracture and deficiency in the trapezius muscle, causing shoulder depression, further diminishing the thoracic outlet, and increasing pressure in the thoracic outlet [4]. After physical therapy as a conservative management, one of the management options for TOS is surgical decompression. The possible complications of such a procedure are numerous, including pneumothorax, hemothorax, chylothorax, bleeding, and nerve injuries such as brachial plexus, phrenic nerve, and thoracic nerve. Despite surgical intervention, the recurrence of symptoms, spanning from 15% to 30%, remains a notable consideration [5,6]. However, a severe cough has never been reported in the literature.
This study aims to report and discuss a case of a severe, intractable cough that developed after nTOS decompression and was managed by injection of local anesthesia. The references have been inspected for reliability, and the report has been written according to the CaReL guidelines [7,8].
Case Presentation
Patient information
A 41-year-old female presented with bilateral upper limb pain and numbness for 15 years, more pronouncedly on the right side. The pain progressed in the last two months.
Clinical findings
The provocative tests for TOS (scalenus muscle tenderness, elevation arm stress test, and Elvey test) were positive.
Diagnostic approach
Electromyography and nerve conduction studies showed nTOS, while chest X-rays, and cervical spine magnetic resonance imaging (MRI) were unremarkable. Doppler ultrasound of the right upper limb showed arterial and venous compression, suggesting nTOS.
Therapeutic intervention
The patient was first advised to do physiotherapy before opting for more invasive interventions, but the patient’s symptoms did not improve after four weeks of physiotherapy. Consequently, the patient was prepared for thoracic outlet decompression (TOD). Under general anesthesia, in the supine position, through a 4-cm supraclavicular incision, anterior, middle, and posterior scalene muscles were resected. The first rib was isolated and resected. Neurolysis was done. The patient was extubated in the operating room. However, in the postoperative period, she developed a severe cough six hours after recovering from the anesthesia. The cough did not respond to the oral and intravenous medications. Two days after the operation, the patient was injected with 120 mg of bupivacaine (5%) into the area of the phrenic nerve in the supraclavicular region-site of the resected scalene anterior muscle [figure 1].
Follow-up and Outcome
The patient responded very well to the injection, and the cough disappeared.
Discussion
After reviewing the genuine literature, we identified several cases of TOD, including a 45-year-old male with right phrenic nerve paralysis post-operation resolving in 3-6 months, a 45-year-old female with postoperative unilateral right diaphragm dysfunction following TOD, and a 40-year-old female with lung herniation after thoracic outlet decompression surgery, which was successfully treated with thoracoscopic reduction and resection, showing no signs of recurrence after 9 months. These cases highlight the varied presentations and outcomes of TOD complications, ranging from respiratory issues to chronic pain, and emphasize the importance of early diagnosis and tailored management strategies [Table 1] [9-11].
|
Author (year)reference |
No. Case |
Age |
Gender |
Presentation |
Previous history |
Clinical examination |
Imaging findings |
Management |
Complication |
Outcome |
|
Heine et al. (1995) [9] |
1 |
45 |
Male |
Numbness and tingling in the right arm, shortness of breath post-operation |
Normal pulmonary reserve |
Post-operation: Dyspnea in the supine position, respiratory rate of 26 breaths per minute, decreased breath sounds in the right hemithorax |
Chest radiograph: Markedly elevated right hemidiaphragm. Fluoroscopic evaluation: Confirmed right phrenic nerve palsy with right diaphragmatic paralysis |
Initial: Restrict activities as tolerated. Follow-up: Continued elevation of the right hemidiaphragm at 3 months, normal pulmonary function tests in the seated position, moderate restrictive defect in the supine position. Six months post-operation: Asymptomatic, normal chest radiograph and fluoroscopic examination, normal pulmonary function tests in both seated and supine positions |
Right phrenic nerve paralysis leading to unilateral diaphragmatic paralysis |
Resolution of phrenic nerve paralysis within 3 to 6 months post-operation |
|
Cain et al. (2021) [10] |
1 |
45 |
Female |
Thoracic outlet syndrome |
Protein C deficiency, recent right thoracic outlet decompression surgery (3 weeks prior), left-sided thoracic outlet decompression surgery (7 months prior) |
dyspnea on exertion
|
Point-of-care ultrasound: decreased movement of right hemidiaphragm compared to left. CT angiography: marked right hemidiaphragm elevation with compressive atelectasis. Sniff test: abnormal motion of right hemidiaphragm during respiration and paradoxical movement during sniff.
|
Not mentioned |
Diagnosed with postoperative unilateral right diaphragm dysfunction.
|
Not mentioned
|
|
Su et al. (2012) [11] |
1 |
40 |
Female |
Chronic left shoulder pain, left upper extremity pain, paresthesias, numbness, decreased functional range of motion of the left shoulder, worsening of symptoms with arm elevation, dyspnea on exertion (4 years after thoracic outlet syndrom decompression)
|
Smoking, previous arthroscopic subacromial decompression and cervical discectomy and fusion, thoracic outlet decompression (supraclavicular approach) 4 years prior
|
Localizing tenderness over the left supraclavicular space, reproducible arm symptoms, visible bulge on the left side of the neck that increased with coughing and exhalation
|
CT scan: Herniation of a large emphysematous bulla from the apex of the left lung into the supraclavicular space, possible compression of the brachial plexus (size: 5.3 x 4.3 x 3.6 cm) |
Thoracoscopic reduction and resection of the herniated lung, pleural flap closure of the defect |
Lung herniation |
Discharged on postoperative day 2 with improvement on chest X-ray. No signs of lung herniation at 9 months follow-up, improvement of arm pain and dyspnea.
|
The shoulder structure predisposes the neurovascular bundle toward the upper limb to compressions during arm abduction at various levels of the thoracic outlet pathway. This neurovascular positional compression may cause pain, discomfort, or problems known as TOS. TOS is classed as a rare disease on both sides of the Atlantic ("NORD®" rare disease database/ORPHANET n°97330), with an estimated frequency of 2.5 to 4.0 cases per 100,000 people per year in the United States and 3 to 4 cases per 100,000 people per year in France. The high frequency of observations in athletes has prompted some scholars to believe that the prevalence of TOS may be significantly higher in these people [12]. In the current study, a 41-year-old female had been experiencing bilateral upper limb discomfort and numbness for 15 years, with more pronounced symptoms on the right side.
Clinical examination involves specific physical maneuvers to identify the type and cause of the syndrome. For nTOS, which is the most common form, physical exams include neck rotation and head tilting, which can elicit symptoms in the contralateral extremity, and the upper limb tension test (ULTT), which is comparable to the straight leg raising test for the lower extremity. Another important maneuver is abducting the arms to 90 degrees in external rotation (90° AER), which typically brings on symptoms within 60 seconds. Imaging studies are crucial for diagnosing TOS. X-rays can reveal cervical ribs or anomalous first ribs, often associated with aTOS. In the case of aTOS, X-rays almost always show these anatomical anomalies. For vTOS, which is indicated by arm swelling, cyanosis, and pain, ultrasound, and venography are used to detect subclavian vein obstruction. Arteriography is used in aTOS to visualize subclavian artery stenosis or aneurysms, often showing emboli arising from these lesions. MRI, or magnetic resonance angiography (MRA), can also be employed to visualize the compression of neurovascular structures [13]. In the current study, electromyography and nerve conduction studies showed nTOS, chest X-rays, and cervical spine MRI were unremarkable. Doppler ultrasound of the right upper limb showed arterial and venous compression, suggesting nTOS.
The initial management of nTOS is by physical therapy, usually for eight weeks. However, if the patient’s condition remains the same or has unsatisfactory improvement after physical therapy, a computed tomography (CT)-guided scalene muscle block can be attempted, which has an important diagnostic value due to its high specificity and sensitivity. Because if the patient's symptoms improve, it indicates that the patient has a high likelihood of benefiting from surgical intervention [14,15]. TOD is the surgical approach utilized for treating TOS. The aim of surgery in TOS is to relieve pressure on the neurovascular structures in the thoracic outlet via surgical decompression. TOD surgery involves the complete resection of the first rib (cartilage to cartilage), transection of the scalene muscles, and thorough neurolysis/venolysis or arteriolysis. Four distinct approaches are available for TOD surgery: transaxillary, supraclavicular, paraclavicular, and infraclavicular. While transaxillary, supraclavicular, and paraclavicular approaches apply to all forms of TOS, the paraclavicular approach is primarily employed in treating vTOS. The infraclavicular approach is not relevant for neurogenic or arterial TOS and is exclusively utilized for vTOS. Each approach has its advantages and limitations, and there is no consensus in the literature on the optimal approach. Therefore, the choice of the surgical approach for TOD should be based on the surgeon's preference and experience [16]. As in the current case, the patient was initially advised to undergo physiotherapy before proceeding with more intrusive measures, but her symptoms did not improve after four weeks of therapy. As a result, the patient was prepared for TOD.
Nonetheless, the surgery might result in various complications, ranging from temporary post-surgery discomfort to lasting disability. The complications vary, including persistent symptoms, injuries to the brachial plexus, phrenic nerve, and long thoracic nerve, leading to partial or temporary paralysis. Additionally, there is a risk of injuries to major blood vessels like the subclavian artery and vein, as well as axillary artery thrombosis. Other potential complications involve hemothorax, pneumothorax, and chylothorax [5]. This patient was free from the expected complications. However, she had a severe, intractable cough postoperatively. The presence of cough post-TODs is very rare, and there has been no other recorded case in the literature to date, at least to the best of our knowledge.
Scott R. Golarz and colleagues showed that supraclavicular decompression for nTOS often involves encountering variations in the anatomy of the phrenic nerve and brachial plexus more frequently. Surgeons operating within the thoracic aperture should have a good understanding of these variant anatomical structures to minimize the risk of nerve injuries, which can lead to severe postoperative complications [17]. A study by Katherine et al. showed that the brachial plexus, phrenic nerve, and long thoracic nerve are located in close proximity to the anterior and middle scalene muscles and the first rib, which are surgically removed to alleviate neurogenic, venous, and arterial thoracic outlet syndrome. Although nerve injury is a relatively rare occurrence, affecting approximately 1% of cases, it can result in significant complications such as hemidiaphragm paralysis, scapular winging, or motor and sensory deficits in the upper extremities. Considering the vital role of the upper extremity, nerve injury can have substantial negative impacts, highlighting the critical importance of understanding nerve anatomy and practicing careful surgical techniques during thoracic outlet syndrome procedures [18].
Phrenic nerve palsy or injury is linked to a reduced cough reflex, and considering the nerve block, the cough is not expected to occur. However, it is essential to note that the efficacy of bupivacaine is temporary, lasting less than 8 hours [19]. Between 40% and 76% of intubated patients experience coughing during the emergence from general anesthesia, and the physiological consequences of peri-extubation coughing can result in notable complications. These complications encompass neck hematoma following thyroidectomy or carotid endarterectomy, wound dehiscence after laparotomy, and intracerebral hemorrhage after intracranial surgery. To mitigate these risks, there is a consensus among authors for a "smooth emergence" strategy, aiming to minimize coughing and, consequently, prevent these potential complications [20]. The current case did not have any of the complications mentioned above. However, she presented with an intractable cough six hours after waking up from anesthesia that was unresponsive to medications. Fortunately, the patient responded well to the injection of local anesthesia into the area of the phrenic nerve. This may indicate neuropraxia (temporary nerve injury or irritation) rather than permanent nerve injury.
Conclusion
A possible complication of surgical decompression for TOS is an intractable cough that can be relieved by the injection of local anesthesia into the area of the phrenic nerve.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Written informed consent was obtained from the patient for publication.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: FHK was a significant contributor to the conception of the study and the literature search for related studies. SHM, RJR, SKA, PMK, MSM and AKG were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. DSH, AAQ, and FA were involved in the literature review, study design, and manuscript writing. SSA, NSS, and FA Literature review, final approval of the manuscript, and processing of the tables. FHK and AKG confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.
