The Hidden Problem of Cross-Reactivity: Challenges in HIV Testing During the COVID-19 Era: A Systematic Review
Abstract
Introduction
Human immunodeficiency virus (HIV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) surface glycoproteins, including shared epitope motifs, show similarities. This may lead to false-positive HIV results due to cross-reactivity between the two viruses. This study presents a systematic review of the published studies on their cross-reactivity.
Methods
A systematic review of the published studies of HIV and SARS-CoV2 cross-reactivity was conducted, the studies that met the following criteria were included: 1) Studies in the English language. 2) Studies in which the title included the required keywords. 3) Studies in which false positive results were achieved and confirmed. 4) Studies investigating the possibility of cross-reactivity between HIV and SARS-CoV2.
Results
A total of 11 studies and 466,140 patients were analyzed. Of the specified sexes, 363,786 (82.1%) of the participants were males. A total of 707 false-positive HIV results were recorded, of which 122 (17.3%) had detectable Coronavirus disease 2019 (COVID-19) antibodies. The remaining 585 (82.7%) false positives were either healthy patients or patients recovered from COVID-19 with no detectable COVID-19 antibodies. Twenty-five distinct tests were used as initial and confirmatory tests for both COVID-19 and HIV. Six (24%) unique fourth-generation HIV antigen/antibody combination tests, six (24%) HIV-specific molecular tests, and four (16%) HIV immunoassays were used.
Conclusion
COVID-19 should be considered a potential cause of false-positive results in HIV tests, due to the cross-reactivity between the antibodies or antigens from both viruses.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic, leading to widespread illness and high mortality rates. This infectious disease exhibits a wide range of clinical manifestations, from no symptoms or mild cases to severe respiratory distress and multi-organ failure [1]. COVID-19 was first identified in individuals exposed to a seafood market in Wuhan City, China, in December 2019. Its rapid spread led the World Health Organization (WHO) to declare it a public health emergency of international concern on January 30, 2020, and it was officially classified as a pandemic on March 11, 2020 [2].
Since the first commercial approval of HIV testing in 1985, significant advancements have been made in the field. However, false positive results are often linked to infections with other pathogens such as Epstein-Barr virus, influenza, and Mycobacterium tuberculosis. Additionally, instances of false positive HIV test results have been reported in conjunction with infections caused by SARS-CoV-2 [3].
Surface glycoproteins of HIV and SARS-CoV-2 exhibit similarities, including shared epitope motifs. As a result, false-positive HIV screening results have been reported in 2020 and 2021 among individuals with acute or previous SARS-CoV-2 infections. False-positive results in HIV enzyme-linked immunosorbent assay (ELISA) tests were also observed during COVID-19 vaccine trials conducted in Australia [4]. These findings emphasize the need to consider recent SARS-CoV-2 infections when interpreting HIV test results. Clinicians should remain vigilant about this association and may need repeated testing to confirm accurate diagnoses. This study aims to add to the available literature through a thorough investigation and comprehensive review of the causes, correlations, and considerations regarding this topic.
Methods
Study design
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5].
Data sources and search strategy
Several strategies were used in conducting the search process, PubMed and Google Scholar were initially utilized using the following keywords: (HIV OR human immunodeficiency virus) AND (COVID-19 OR SARS-CoV2) AND (Cross-reactivity) AND (False-positive). Citations in the retrieved studies were also utilized to recover more papers. The AI tools “Perplexity” and “Consensus” were also used to strengthen the search process to find similar documents.
Eligibility criteria
The studies with the following specifications were included in the study: 1) Studies in the English language. 2) Studies in which the title included the required keywords. 3) Studies in which false positive results were achieved and confirmed. 4) Studies investigating the possibility of cross-reactivity between HIV and COVID-19. Studies published in non-peer-reviewed journals [6] and those failing to meet the inclusion criteria were excluded from the review.
Selection and extraction of data
The titles and abstracts of identified studies were first screened, followed by a thorough full-text review to assess eligibility. Key data, including study design, number of patients, patient demographics, COVID-19 status, HIV status, testing techniques, and test results were extracted from the included studies.
Data analysis
Data was analyzed using Microsoft Excel (2019) to collect and organize the extracted data. The Statistical Package for Social Sciences (SPSS) version 27.0 was employed for the analysis, specifically for descriptive statistics. The results are presented as frequencies, percentages, medians, and mean with standard deviations.
Results
A total of 43 studies were retrieved from the search, of which four were excluded, before any screening due to being unretrievable, and one study was excluded for being written in non-English language. During the initial screening, the titles of 19 studies didn’t meet the inclusion criteria. Upon screening, six more studies were excluded as their abstracts didn’t meet the inclusion criteria. After a thorough assessment for eligibility, two more studies were removed because they were from non-peer-reviewed journals. Ultimately, 11 studies were included and analyzed [3,4,7-9,12,15-19] (Figure 1).
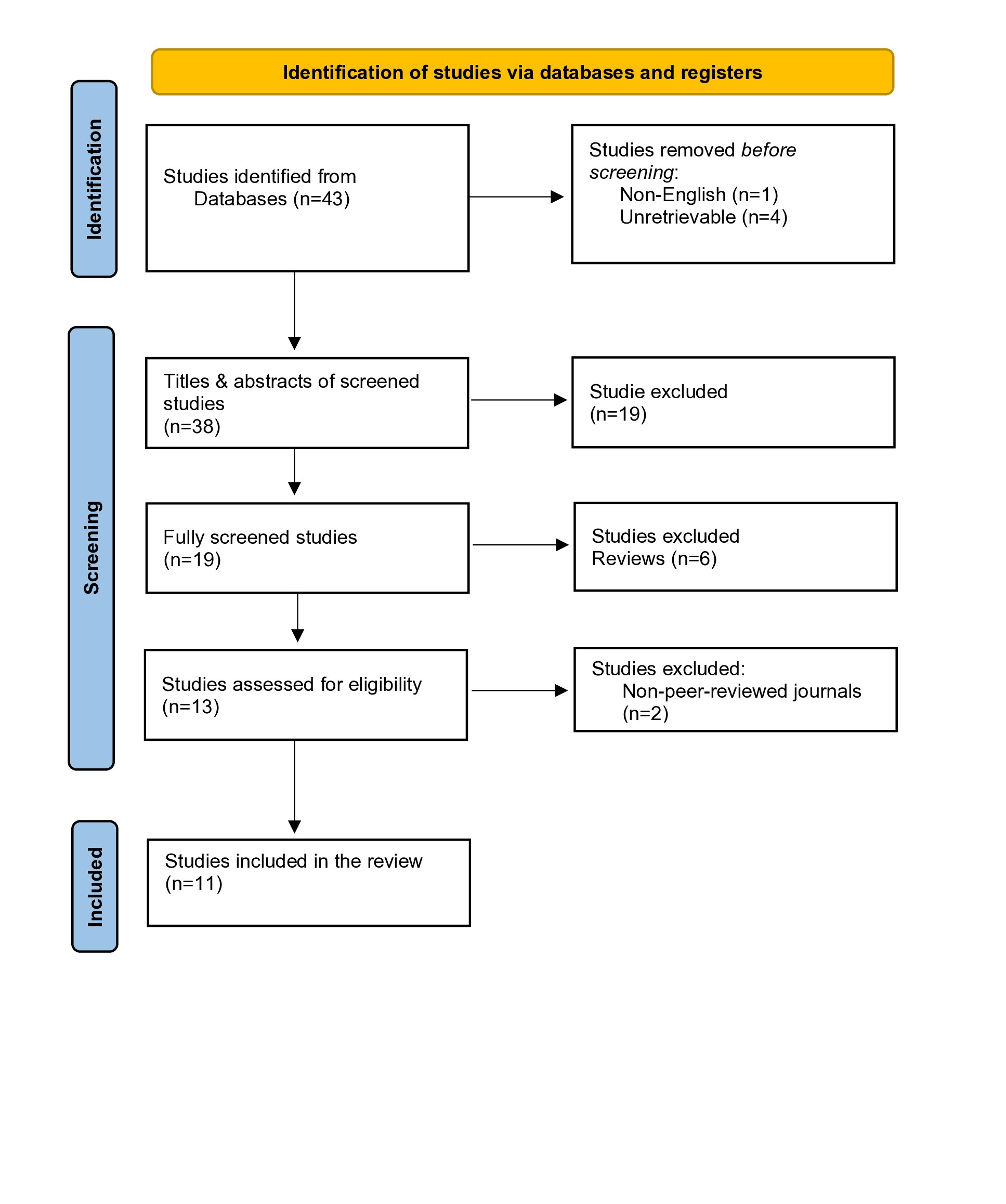
A total of 466,140 patients were analyzed. Of the specified sexes, 363,786 (82.14%) of the participants were males. A total of 707 false-positive HIV results were recorded, of which 122 (17.3%) had detectable COVID-19 antibodies. The remaining 585 (82.7%) false positives were either healthy patients or recovered from COVID-19 with no detectable antibodies. One case of false-positive COVID-19 in an acute HIV infection was also recorded (Table 1).
|
Author/Year |
Study design |
Number of patients |
Age* |
Sex |
COVID-19 status |
HIV status |
Initial testing technique |
Confirmatory testing technique |
Final Results |
|
|
M |
F |
|||||||||
|
Alfie et al./ 2023 [4] |
Cohort |
921 |
Median age 41 (IQR 32-54)
|
277 |
397 |
Detectable Covid antibodies = 674 |
True +ve in 3 patients -ve in 671 patients |
Genscreen Ultra HIV Ag-Ab & COVIDAR kit |
ELISA, RecomLine HIV-1 & HIV-2 IgG, &Abbott m2000 RealTime PCR |
False +ve HIV in 12 (1.8%) patients |
|
43 (IQR 34-56)
|
90 |
110 |
Previously diagnosed with COVID with no detectable antibody = 200 |
-ve |
No false +ve HIV results |
|||||
|
42 (IQR 36-57) |
18 |
29 |
Vaccinated = 47 |
-ve |
No false +ve HIV results |
|||||
|
Shallal et al./ 2022 [9] |
Cross-sectional |
23,278 |
N/A |
N/A |
Total=167 +ve = 12 -ve = 155 |
True +ve in 167 patients |
Elecsys HIV Duo & PCR test |
HIV-1 and 2 antibody tests & Quantitative HIV RNA test |
No +ve HIV tests |
|
|
Total=70 +ve= 16 -ve = 54 |
False +ve HIV in 70 patients, of which 16 (22.9%) were +ve for Covid. |
|||||||||
|
Total=23,041 +ve = 0 -ve = 23,041 |
No false +ve HIV tests |
|||||||||
|
Hayat et al./ 2021 [15] |
Cross-sectional |
2,593 |
Median age
21.5 |
2,361 |
232 |
Recovered with detectable antibodies |
True +ve in one patient |
Electrochemiluminescence immunoassay & polymerase chain reaction |
Line immunoassay |
False +ve HIV in 68 (1.84%) donations |
|
407,363 |
27 |
350,724 |
56,639 |
Healthy |
True +ve in 49 patients |
False +ve HIV in 461 donations |
||||
|
Gudipati et al./ 2023 [8] |
Cross-sectional |
31,910 |
Mean age 37.13 |
10,295 |
21,615 |
True +ve in 229 patients |
True +ve in 248 patients |
SARS-CoV-2 Real-Time PCR Test & HIV Fourth-Generation Ag/Ab Assay |
HIV-1/HIV-2 Antibody Differentiation Immunoassay & HIV-1 Nucleic Acid Amplification Test |
False +ve HIV in 87 patients of which 17 (19.54%) were +ve for Covid |
|
Elsner et al./2023 [16] |
Cohort |
65 |
Median age 51 (IQR 19) |
13 |
42 |
Previously diagnosed with covid |
-ve |
Elecsys HIV combi PT & Architect HIV Ag/Ab Combo |
INNO-LIA HIV I/II Score |
No false +ve HIV results |
|
1 |
32 |
|
1 |
+ve |
-ve |
Elecsys HIV combi PT, INNO-LIA HIV I/II Score |
Architect HIV Ag/Ab Combo, INNO-LIA HIV I/II Score, HIV-1 qPCR |
Repeated False +ve HIV for 3 months with subsequent Resolution |
||
|
Hakobyan et al./2023 [17] |
Case report |
2 |
69 |
1 |
|
+ve |
-ve |
Fourth-generation HIV combination test |
ELISA, HIV-1 genotype testing, Western blot & HIV integrase genotype test |
False +ve HIV |
|
80 |
1 |
|
+ve |
-ve |
Fourth-generation HIV combination test |
ELISA, Viral load test |
False +ve HIV |
|||
|
Tan et al./ 2020 [18] |
Case report |
2 |
Early 20s |
1 |
|
+ve |
-ve |
Chemiluminescent immunoassay |
VIDAS HIV duo assay & MP Biomedicals HIV immunoblot |
False +ve HIV |
|
Early 70s |
1 |
|
+ve |
-ve |
Chemiluminescent immunoassay |
VIDAS HIV duo assay & MP Biomedicals HIV immunoblot |
False +ve HIV |
|||
|
Srivastava et al./2022 [19] |
Case report |
2 |
69 |
1 |
|
+ve |
-ve |
HIV DUO ULTRA, 4th generation assay |
TRI-DOT Rapid HIV flow-through test |
False +ve HIV |
|
9 |
1 |
|
+ve |
-ve |
HIV DUO ULTRA, 4th generation assay |
TRI-DOT Rapid HIV flow-through test |
False +ve HIV |
|||
|
Salih et al./ 2021 [7] |
Case report |
1 |
32 |
|
1 |
+ve |
-ve |
HIV immunoassay test |
RN PCR |
False +ve HIV |
|
Balasubramanian et al./ 2023 [3] |
Case report |
1 |
20 |
1 |
|
+ve |
-ve |
4th Generation HIV 1 and 2 antibody/antigen testing |
HIV antibody testing |
False +ve HIV |
|
Yamaniha et al./2021 [12] |
Case report |
1 |
39 |
1 |
|
-ve |
+ve |
Rapid Antigen Test for SARS-CoV-2 & Rapid Antigen/Antibody Test for HIV |
Real-Time Polymerase Chain Reaction, Chemiluminescent Immunoassay, Western Blot Assay & HIV-RNA |
False +ve Covid in a patient with acute HIV infection |
|
*Age was not given in a uniform manner among the different studies. N/A: not applicable, +ve: Positive, -ve: Negative, Cp:Convalescent plasma |
||||||||||
Twenty-five distinct tests were used as initial and confirmatory tests for both COVID-19 and HIV. Six (24%) unique fourth-generation HIV antigen/antibody combination tests, six (24%) HIV-specific molecular tests, and four (16%) HIV-specific antibody tests were used (Table 2).
|
Variables |
Frequency (%) |
|
Sex* Male Female |
Number of patients (442,852) 363,786 (82.1%) 79,066 (17.9%) |
|
Age* Combined mean Combined median Age variance |
Number of patients (442,852) 46.89 ± 8.48 38.65 19.94 |
|
Testing techniques HIV Antibody/Antigen (4th Generation) Test HIV-Specific Molecular Tests HIV Antibody-Specific Tests HIV Immunoassays Rapid tests SARS-CoV-2-Specific Tests HIV Differentiation Tests |
Total unique tests (25)
6 (24%)
6 (24%) 4 (16%) 4 (16%) 2 (8%) 2 (8%) 1 (4) |
|
False-positive HIV results Detectable COVID-19 antibodies Idiopathic false-positives Idiopathic false-positive HIV results 4TH Generation HIV Ag/Ab Test Enzyme-linked immunosorbent assay |
Total (707) 122 (17.3%) 585 (82.7%) Total (585) 124 (21.2%) 461 (78.8%) |
| *The sex and age of 23,278 patients from Shallal et al. were not mentioned | |
Discussion
As a systemic illness, COVID-19 affects multiple body systems, and a minority of patients may also develop additional microbial co-infections that worsen their condition. Approximately 7.2% of cases are reported to involve co-infections with other bacterial, fungal, or viral pathogens, which can influence both patient outcomes and treatment strategies. However, instances of false-positive results for co-infections and misdiagnoses have been documented in the context of COVID-19. For example, cross-reactivity between SARS-CoV-2 and certain pathogens, such as the Dengue virus, has been occasionally reported in the literature [7]. During the 2003 severe acute respiratory syndrome (SARS) pandemic, it was demonstrated through sequence analysis that the viral proteins of HIV and SARS-CoV-1 shared sequence motifs that contributed to forming their active conformation [8]. In the current review, 17.3% of the false positives were of patients with detectable COVID-19 antibodies, showing a high possibility of cross-reactivity. Shallal et al. analyzed 23,278 medical charts and found that false-positive HIV was significantly higher in patients with COVID-19 [9].
Alfie et al. showed that compared to the Centers for Disease Control and Prevention (CDC) rate of false positive HIV screenings, which is 0.4%, the rate of false positives is significantly higher when COVID-19 antibodies are detectable, at 1.8%. When considering samples only from people previously diagnosed with COVID-19, the rate is again significantly higher at 1.4% [4]. In a cross-sectional study of 31,910 medical records, Gudipati et al. showed that After accounting for all covariates, only false-positive HIV was significantly linked to COVID-19 [8] .
While exploring the cross-reactivity of antibodies targeting HIV-1 with the SARS-CoV-2 spike protein, Mannar et al. identified 2G12, PGT128, and PGT126, three glycan-reactive antibodies that exhibited various levels of cross-reactivity with SARS-CoV-2 spike protein [10]. In a similar investigation, Perween et al. demonstrated that antibodies targeting the SARS-CoV-2 spike protein could cross-react with HIV-1 envelope proteins, particularly gp41; however, these antibodies did not neutralize HIV-1. Conversely, antibodies against HIV-1 envelope protein gp140 also exhibited cross-reactivity with SARS-CoV-2 spike protein but lacked neutralizing capability against SARS-CoV-2 [11]. This bidirectional cross-reactivity was further illustrated by a case reported by Yamaniha et al. , which reported a case of false positive COVID-19 in a 39-year-old male with acute HIV infection [12]. Zhang et al. contributed to this discourse by confirming that 4 specific insertions in the spike protein of SARS-CoV-2 share similarities with HIV-1 proteins [13]. They also observed that the spike protein contained short insertions made up of 6-8 amino acid segments. However, they posited that while these similarities suggest potential cross-reactivity between antigens of both viruses, they may also result from convergent evolution or shared structural features across different viral families. In the current review, 585 (82.7%) of the false positives were idiopathic, of which 124 (21.2%) were tested with 4th generation HIV assays, which work by utilizing distinct, simultaneous reactions to identify HIV antigen (p24) and HIV-1/2 antibodies. The system converts cut-off index (COI) values into qualitative results, reporting them as nonreactive (COI < 1.0) or reactive (COI ≥ 1.0) [8]. Zhang et al. suggested that due to the nature of the test, an exact amino acid sequence homology to HIV is not required to yield a false positive test result, it requires only enough antigenic similarity for a detectable amount of false signal [13] . The absence of strict homology and the short length may help to explain the idiopathic occurrence of false positive HIV results in some individuals. While antigenic homology may play a key role, the connection to SARS-CoV-2 antigens remains unclear. Yang et al. published the results of an HIV screening program that used a 4th generation HIV assay, they reported that out of the 578 participants who screened positive for HIV, 13.3% were positive for both antigen and antibody, 77.7% were positive for antibodies only, and 9.0% were positive for antigens only, making it important for more research to be conducted to build models that offer empirical evidence to further support these hypotheses in future research [14].
While conducting the review, certain limitations were identified. Firstly, the variation in data presentation across the papers hindered the ability to maintain uniformity when finalizing the data. The retrospective nature of the studies made it difficult to create a true correlation between the variables.
Conclusion
Human immunodeficiency virus and COVID-19 exhibit cross-reactivity at several levels. Although the exact mechanisms and models have not been established yet, the findings highlight the importance of considering recent SARS-CoV-2 infections when interpreting HIV test results and implementing confirmatory tests to achieve true results.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: BAA, RQS and DAH significantly contributed to the study's conception and the literature search for related studies. MMA, SHM, SLE, and REA were involved in the literature review, manuscript writing, and data analysis and interpretation. RAA, TSH, NHM, KKM, SJI, DQH and BHB were involved in the literature review, the study's design, and the manuscript's critical revision. BAA and MMA confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: Perplexity AI v3.2.0 and Consensus AI were used in the literature review, the author assumes full responsibility for the content of the paper.
Data availability statement: Not applicable.
References
- Qadir F. I., Kakamad F. H., Abdullah I. Y., Abdulla B. A., Mohammed S. H., Salih R. Q., et al. The relationship between CT severity infections and oxygen saturation in patients infected with COVID-19, a cohort study. Annals of Medicine and Surgery. 2022; 76: 103439. doi:10.1016/j.amsu.2022.103439
- Ahmad S. A., Salih B. K., Hama Hussein K. F., Mikael T. M., Kakamad F. H., Salih A. M. Aseptic meningoencephalitis after COVID-19 vaccination: A case report. Annals of Medicine and Surgery. 2021;71: 103028. doi:10.1016/j.amsu.2021.103028
- Balasubramanian A, Singh D, Lahey T. COVID-19 infection and a repeated false positivity effect in HIV testing: A case report. IDCases. 2023 ;34:e01908. doi:10.1016/j.idcr.2023.e01908
- Alfie LG, Longueira YS, Pippo M, Cruces L, Quiroga MF, Turk G, Laufer N. Increased risk of false-positive HIV ELISA results after COVID-19. Aids. 2023;37(6):947-50. doi:10.1097/QAD.0000000000003507
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021 Apr; 88:105906. doi:10.1136/bmj.n71
- Kakamad FH, Abdalla BA, Abdullah HO, Omar SS, Mohammed SH, Ahmed SM, et al. Lists of predatory journals and publishers: a review for future refinement. European Science Editing. 2024; 50: 118119. doi:10.3897/ese.2024.e118119
- Salih RQ, Salih GA, Abdulla BA, Ahmed AD, Mohammed HR, Kakamad FH et al. False-positive HIV in a patient with SARS-CoV-2 infection; a case report. Annals of Medicine and Surgery. 2021;71. doi:10.1016/j.amsu.2021.103027
- Gudipati S, Shallal A, Peterson E, Cook B, Markowitz N. Increase in false-positive fourth-generation human immunodeficiency virus tests in patients with coronavirus disease 2019. Clinical Infectious Diseases. 2023;77(4):615-9. doi:10.1093/cid/ciad264
- Shallal A, Gudipati S, Peterson E, Cook B, Tibbetts R, Markowitz N. Increase in false positive 4th generation HIV tests in patients with COVID-19 disease. Proceedings of the CROI. 2022. doi:N/A
- Mannar D, Leopold K, Subramaniam S. Glycan reactive anti-HIV-1 antibodies bind the SARS-CoV-2 spike protein but do not block viral entry. Scientific Reports. 2021 ;11(1):12448.doi:10.1038/s41598-021-91746-7
- Perween R, PraveenKumar M, Shrivastava T, Parray HA, Singh V, Singh S et al. The SARS CoV-2 spike directed non-neutralizing polyclonal antibodies cross-react with Human immunodeficiency virus (HIV-1) gp41. International Immunopharmacology. 2021 ;101:108187. doi:10.1016/j.intimp.2021.108187
- Yamaniha K, Kinjo T, Akamine M, Setoguchi M, Tateyama M, Fujita J. False-positive for SARS-CoV-2 antigen test in a man with acute HIV infection. Journal of Infection and Chemotherapy. 2021;27(7):1112-4. doi:10.1016/j.jiac.2021.04.011
- Zhang C, Zheng W, Huang X, Bell EW, Zhou X, Zhang Y. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. Journal of proteome research. 2020;19(4):1351-60. doi:10.1021/acs.jproteome.0c00129
- Yang M, Yang W, Shi W, Tao C. Clinical application evaluation of Elecsys® HIV duo assay in southwest China. Frontiers in Cellular and Infection Microbiology. 2022;12:877643. doi:10.3389/fcimb.2022.877643
- Hayat L, Beker C, Karaca A, Hafizoglu N, Kinik K, Yilmaz FA. Antibody false positivity among COVID-19 convalescent plasma donors: a comparative study from the Turkish Red Crescent Blood Center. Haseki Tip Bulteni. 2021;59(5). doi:10.4274/haseki.galenos.2021.7383
- Elsner C, Appeltrath GA, Konik M, Parreuter J, Broecker-Preuss M, Krawczyk A et al. False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies. Viruses. 2023;15(5):1161. doi:10.3390/v15051161
- Hakobyan N, Yadav R, Abaza K, Friedman A. False-positive human immunodeficiency virus results in COVID-19 patients. Cureus. 2023;15(1). doi:10.7759/cureus.34096
- Tan SS, Chew KL, Saw S, Jureen R, Sethi S. Cross-reactivity of SARS-CoV-2 with HIV chemiluminescent assay leading to false-positive results. Journal of clinical pathology. 2021;74(9):614-. doi:10.1136/jclinpath-2020-206942
- Srivastava S, Singh P, Malhotra R, Mathur P. False-positive human immunodeficiency virus reactivity in COVID patients: a word of caution. Journal of Global Infectious Diseases. 2022;14(1):43-4. doi:10.4103/jgid.jgid_226_21

This work is licensed under a Creative Commons Attribution 4.0 International License.

