Microwave Ablation with or Without Chemotherapy in Management of Non-Small Cell Lung Cancer: A Systematic Review
Abstract
Introduction
Microwave ablation (MWA) has emerged as a minimally invasive treatment for patients with inoperable non-small cell lung cancer (NSCLC). However, whether it is more effective as a standalone treatment or in combination with chemotherapy warrants further investigation. This systematic review assesses the efficacy and safety of MWA as a standalone treatment and in combination with chemotherapy in managing NSCLC.
Methods
Studies were included if MWA was used either as a standalone treatment or combined with chemotherapy for managing NSCLC, regardless of whether chemotherapy was administered before or after MWA.
Results
The patient cohort included 928 patients. In 63.8% of the cases, MWA was used alone, and in 36.2% with chemotherapy. Complications from MWA alone were higher (59.29% vs. 32.74%). The tumor stage in 52.36% of the cases who underwent MWA alone was stage I; however, it was the IV stage in 82.44% of the cases who underwent MWA combined with chemotherapy. Patients with available data and treated with MWA alone experienced higher local progression (26% vs. 18.5%), distant recurrence (51.5% vs. 38.5%), and both local and distant recurrence (10.8% vs. 2.6%). Reported complete response was 88.6% among cases that underwent MWA alone. While it was 78.0% in those who underwent combined MWA and chemotherapy. The median overall survival was higher in the MWA alone group (24.9 to 69.6 months vs. 21.3 to 23.90 months).
Conclusion
MWA combined with chemotherapy may represent a more effective option, with a slightly similar treatment response, reducing the risk of recurrence and minimizing complications.
Introduction
Lung cancer is the leading cause of cancer-related mortality among both men and women worldwide [1,2]. It primarily consists of two main types: non-small cell lung cancer (NSCLC), which comprises 85% of cases, and small cell lung cancer (15%). The World Health Organization classifies NSCLC into three main subtypes: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [1]. NSCLC has one of the lowest five-year survival rates, hovering around 20% [3]. Cigarette smoking is the primary risk factor for lung cancer, responsible for 85% to 90% of cases. The risk is closely linked to smoking extent and exposure to carcinogens like asbestos, ionizing radiation, environmental toxins, and certain metals. Other risk factors include pulmonary fibrosis and alcohol consumption [1]. Occupational exposures, particularly to carcinogens like crystalline silica, asbestos, and radioactive materials, significantly increase lung cancer risk. In addition, familial clustering suggests a hereditary component to the disease [4]. Although smoking remains the leading cause, 12% of lung cancer cases are non-smokers, with higher rates in women [1,5]. The most common symptom of the disease is cough, followed by hemoptysis and chest pain [6]. Lung cancer is often diagnosed at intermediate or advanced stages due to its low early diagnostic rate, high malignancy, and complex biological characteristics [7]. However, low-dose computed tomography screening has improved early detection rates [8]. Surgical resection is the preferred treatment for early-stage NSCLC when the disease is operable. However, despite advancements in sub-lobar resection techniques to preserve lung function, more than 25% of patients with early-stage NSCLC are unable to undergo surgery due to factors like poor cardiopulmonary function, anatomical challenges, failure of conventional therapies, or personal choice. For these patients, non-surgical options such as stereotactic ablative radiotherapy, targeted therapies, and thermal ablation are typically recommended [6,8-10]. Thermal ablation therapies use various approaches to destroy cancer cells through heat or cold. In recent years, microwave ablation (MWA) has emerged as a viable alternative, offering outcomes comparable to lobectomy [8,9]. To our knowledge, no systematic review has been conducted on the effect of MWA alone or in combination with chemotherapy in managing NSCLC. This systematic review aims to evaluate the efficacy and safety of both treatment regimens in managing NSCLC.
Methods
Study design
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and search strategy
A systematic search on PubMed and Google Scholar was conducted to identify relevant English-language studies using MWA with chemotherapy or MWA alone in treating NSCLC. The search utilized the keywords "microwave ablation," "chemotherapy," "non-small cell lung cancer," "adenocarcinoma," "squamous cell carcinoma," and "large cell carcinoma."
Eligibility criteria
Studies were included if MWA was used either as a standalone treatment or combined with chemotherapy for managing NSCLC, regardless of whether chemotherapy was administered before or after MWA. Excluded studies included abstracts, retracted papers, case reports, reviews, and publications in predatory journals [11].
Study selection and data extraction
The following data were extracted from each eligible article: author, year of publication, study design, sample size, patient demography, tumor characteristics, management, characteristics of the MWA (frequency, antenna length, anesthesia type, power, ablation time), the chemotherapy drug, complications, and the outcomes.
Statistical analysis
The extracted data were collected in a Microsoft Excel sheet (2021) and then transferred into the Statistical Package for the Social Sciences software (version 27). Qualitative analysis was conducted, and the data were presented as frequency, percentage, mean with standard deviation, and median with range.
Results
Study selection
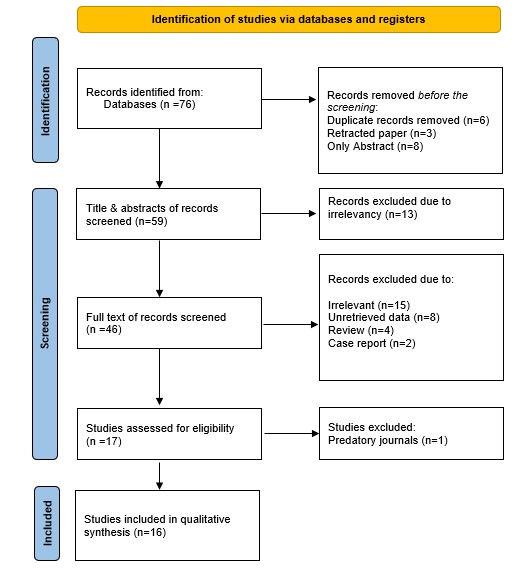
A total of 76 studies were identified through the search. Before screening, 17 studies were excluded due to duplication (n=6), retraction (n=3), and being available only as abstracts (n=8), leaving 59 studies for title and abstract screening. At this stage, 13 irrelevant studies were excluded. Irrelevant studies were those utilizing treatment modalities other than MWA with or without chemotherapy or where patients received other treatments before or after the primary intervention. Consequently, 46 studies underwent full-text screening, which led to the exclusion of 29 studies due to irrelevancy (n=15), unretrievable data (n=8), review articles (n=4), and case reports (n=2). Then, another study was excluded for being published in a predatory journal. Ultimately, 16 studies involving 928 cases met the eligibility criteria and were included [7-9,12-24] (Figure 1). The majority of included studies were cohort studies (n=14), along with two randomized controlled trials (RCTs) (Tables 1 and 2).
|
Author, year [Reference] |
Study design |
No. of cases |
Mean age) |
Gender |
Mean tumor size (cm) |
Tumor staging |
Tumor type |
Location (Lobe) |
Management |
Guidance |
Frequency (MHz) |
Antenna length (Max, mm) |
Antenna diameter (Max, G) |
Active tip (mm) |
Anesthesia |
||||||||||
|
M |
F |
I |
II |
III |
IV |
ADC |
SCC |
Other |
U&M |
L |
CS |
LA |
LA + CS |
LA + IV |
|||||||||||
|
Shan et al. 2021 [7] |
RCT |
67 |
61.5 |
46 |
21 |
3.8 |
0 |
0 |
0 |
67 |
29 |
38 |
N/A |
N/A |
N/A |
MWA + Chemo. |
CT |
N/A |
N/A |
N/A |
N/A |
0 |
67 |
0 |
0 |
|
Wu et al. 2024 [8] |
Cohort |
55 |
59.75 |
40 |
15 |
2.89 |
55 |
0 |
0 |
0 |
28 |
23 |
4 |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
19 |
N/A |
55 |
0 |
0 |
0 |
|
Han et al. 2019 [9] |
Cohort |
63# |
82.1 |
40 |
23 |
N/A |
N/A |
N/A |
N/A |
N/A |
47 |
17 |
1 |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
18 |
15 |
0 |
63 |
0 |
0 |
|
Lv et al. 2023 [12] |
Cohort |
118 |
N/A |
69 |
49 |
N/A |
N/A |
N/A |
N/A |
N/A |
94 |
N/A |
N/A |
73 |
45 |
MWA |
CT |
2450 |
180 |
18 |
N/A |
0 |
118 |
0 |
0 |
|
Xu et al. 2023 [13] |
Cohort |
33* |
68.4 |
19 |
14 |
4.4 |
6 |
3 |
7 |
1 |
25 |
7 |
1 |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
18 |
15 |
0 |
0 |
0 |
33 |
|
Li et al. 2023 [14] |
Cohort |
19 |
71.42 |
15 |
4 |
2.06 |
19 |
0 |
0 |
0 |
6 |
12 |
1 |
14 |
5 |
MWA |
CT |
2450 |
180 |
18 |
5 |
0 |
19 |
0 |
0 |
|
Hu et al. 2021 [15] |
Cohort |
68 |
83.1 |
44 |
24 |
2.3 |
68 |
0 |
0 |
0 |
41 |
24 |
N/A |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
17 |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Das et al. 2019 [16] |
Cohort |
56 |
59.1 |
34 |
22 |
2.9 |
0 |
0 |
32 |
24 |
43 |
10 |
3 |
37 |
19 |
MWA |
CT |
N/A |
N/A |
20 |
N/A |
0 |
56 |
0 |
0 |
|
Wei et al. 2019 [17]** |
Cohort |
18 |
74 |
9 |
9 |
3.3 |
0 |
0 |
8 |
10 |
13 |
N/A |
N/A |
10 |
8 |
MWA |
CT |
2450 |
180 |
20 |
N/A |
0 |
18 |
0 |
0 |
|
Wei et al. 2019 [17]** |
Cohort |
36 |
76 |
21 |
15 |
4.3 |
0 |
0 |
16 |
20 |
28 |
N/A |
N/A |
24 |
12 |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
36 |
0 |
0 |
|
Wang et al. 2018 [18] |
Cohort |
46 |
N/A |
22 |
24 |
N/A |
46 |
0 |
0 |
0 |
18 |
21 |
7 |
N/A |
N/A |
MWA |
CT |
N/A |
200 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Yao et al. 2018 [19] |
Cohort |
54 |
56.65 |
37 |
17 |
3.01 |
54 |
0 |
0 |
0 |
27 |
16 |
11 |
30 |
24 |
MWA |
CT |
N/A |
N/A |
N/A |
N/A |
54 |
0 |
0 |
0 |
|
Yang et al. 2014 [20] |
Cohort |
47 |
69.4 |
30 |
17 |
N/A |
47 |
0 |
0 |
0 |
28 |
13 |
N/A |
N/A |
N/A |
MWA |
CT |
2450 |
180 |
20 |
15 |
0 |
47 |
0 |
0 |
|
Liu et al. 2013 [21] |
Cohort |
15 |
71.25 |
11 |
4 |
2.55 |
15 |
0 |
0 |
0 |
N/A |
N/A |
N/A |
10 |
5 |
MWA |
CT |
2450 |
140 |
N/A |
16 |
0 |
0 |
15 |
0 |
|
Wei et al. 2020 [22] |
RCT |
148 |
59 |
96 |
52 |
3.6 |
0 |
0 |
31 |
117 |
116 |
N/A |
N/A |
N/A |
N/A |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
148 |
0 |
0 |
|
Wei et al. 2015 [23] |
Cohort |
46 |
58.5 |
27 |
19 |
3.7 |
0 |
0 |
8 |
38 |
36 |
N/A |
N/A |
32 |
14 |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
46 |
0 |
0 |
|
Wei et al. 2014 [24] |
Cohort |
39 |
57 |
22 |
17 |
3.84 |
0 |
0 |
4 |
35 |
27 |
N/A |
N/A |
28 |
11 |
MWA + Chemo. |
CT |
2450 |
180 |
20 |
N/A |
0 |
39 |
0 |
0 |
|
Author, year [Reference] |
Power (Max, W) |
Ablation time (Max, Min) |
Ablation beyond margin (max, mm) |
Chemotherapy drug |
Complications |
Outcome |
OS (median, month) |
PFS (median, month) |
follow up (median, month) |
D |
A |
||||||||||||
|
Pem |
Doc |
Pac |
Gem |
Tig |
Cis |
Carbo |
Neda |
PTX |
PE |
Other |
LP |
R |
CR |
PR |
|||||||||
|
Shan et al. 2021 [7] |
80 |
20 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
6 |
N/A |
36 |
N/A |
N/A |
8 |
28 |
N/A |
4.5 |
6 |
N/A |
N/A |
|
Wu et al. 2024 [8] |
40 |
N/A |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
18 |
2 |
27 |
N/A |
31 |
N/A |
N/A |
69.6 |
N/A |
55.2 |
N/A |
N/A |
|
Han et al. 2019 [9] |
80 |
20 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
15 |
2 |
7 |
14 |
6 |
N/A |
N/A |
50.0 |
N/A |
21 |
N/A |
N/A |
|
Lv et al. 2023 [12] |
70 |
N/A |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
47 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Xu et al. 2023 [13] |
40 |
16.9 |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
4 |
4 |
15 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Li et al. 2023 [14] |
40 |
15 |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
5 |
2 |
18 |
N/A |
7 |
N/A |
N/A |
25 |
N/A |
20.4 |
14 |
5 |
|
Hu et al. 2021 [15] |
40 |
8 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
16 |
2 |
N/A |
N/A |
23 |
N/A |
N/A |
N/A |
N/A |
45 |
20 |
48 |
|
Das et al. 2019 [16] |
80 |
10 |
5 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
23 |
14 |
36 |
15 |
48 |
N/A |
N/A |
27.5 |
11.0 |
19.5 |
N/A |
N/A |
|
Wei et al. 2019 [17]** |
70 |
N/A |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
15 |
8 |
N/A |
N/A |
N/A |
15 |
3 |
24.9 |
14.1 |
N/A |
N/A |
N/A |
|
Wei et al. 2019 [17]** |
100 |
33 |
10 |
23 |
2 |
5 |
3 |
3 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
27 |
9 |
21.6 |
4.8 |
N/A |
N/A |
N/A |
|
Wang et al. 2018 [18] |
N/A |
N/A |
5 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
7 |
4 |
N/A |
N/A |
N/A |
46 |
N/A |
32.7 |
N/A |
N/A |
N/A |
N/A |
|
Yao et al. 2018 [19] |
N/A |
10 |
10 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
7 |
N/A |
11 |
N/A |
39 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Yang et al. 2014 [20] |
N/A |
8 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
30 |
16 |
42 |
13 |
13 |
N/A |
N/A |
33.8 |
N/A |
30 |
26 |
21 |
|
Liu et al. 2013 [21] |
110 |
7 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
1 |
5 |
N/A |
9 |
2 |
N/A |
N/A |
12 |
N/A |
N/A |
|
Wei et al. 2020 [22] |
100 |
8 |
10 |
97 |
23 |
8 |
20 |
N/A |
40 |
19 |
89 |
N/A |
N/A |
N/A |
27 |
N/A |
132 |
N/A |
N/A |
10.3 |
13.1 |
N/A |
N/A |
|
Wei et al. 2015 [23] |
80 |
N/A |
5 |
19 |
16 |
4 |
7 |
N/A |
N/A |
N/A |
N/A |
18 |
15 |
9 |
9 |
N/A |
39 |
7 |
23.9 |
10.9 |
21 |
16 |
30 |
|
Wei et al. 2014 [24] |
70 |
11 |
5 |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
12 |
7 |
7 |
7 |
16 |
N/A |
33 |
21.3 |
8.6 |
11.2 |
9 |
30 |
|
RCT: Randomized clinical trials, N/A: Not available, M: Male, F: Female, ADC: Adenocarcinoma, SCC: Squamous cell carcinoma, U: Upper, M: Middle, L: Lower, MWA: Microwave ablation, Chemo.: Chemotherapy, CT: Computed tomography, MHz: Megahertz, Max: Maximum, mm: millimeter, G: Gauge, CS: Conscious sedation, LA: Local anesthesia, IV: Intravenous, W: Watt, Min: Minute, Pem: Pemetrexed, Doc: Docetaxel, Pac: Paclitaxel, Gem: Gemcitabine, Tig: Tigio, Cis: Cisplatin, Carbo: Carboplatin, Neda: Nedaplatin, PTX: Pneumothorax, PE: Pleural effusion, LP: Local progression, R: Recurrence, CR: Complete response, PR: Partial response, OS: Overall survival, PFS: Progression-free survival, D: Death, A: Alive. *Tumor staging was available for only 17 cases. #For 63 cases, 65 tumors were diagnosed. **The same study with two arms, one with only MWA and the other with MWA combined chemotherapy. |
|||||||||||||||||||||||
Patients and Tumor Characteristics
The total sample size was 928 cases, with a gender distribution of 582 males (62.70%) and 346 females (37.30%). The mean age of the patients was 66.80 ± 8.87 years, and the mean tumor size was 3.33 ± 0.73 cm. Most of the cases were in stage IV (33.62%) and stage I (33.41%). Adenocarcinoma was the most common tumor type (65.52%), followed by squamous cell carcinoma (19.50%). The tumors were commonly located in the upper and middle lobes of the lungs (27.80%) (Table 3).
|
Variables |
Frequency & Percentages |
||
|
Overall |
MWA alone group |
MWA plus Chemotherapy group |
|
|
No. of cases |
928 |
592 (63.80%) |
336 (36.20%) |
|
Age (mean ± SD, year) |
66.80 ± 8.87 |
69.52 ± 9.07 |
62.28 ± 6.96 |
|
Study design Cohort Randomized clinical trial |
15 (88%) 2 (12%) |
12 (80%) 0 (0%) |
3 (20%) 2 (100%) |
|
Gender Male Female |
582 (62.70%) 346 (37. 30%) |
370 (62.50%) 222 (37.50%) |
212 (63.10%) 124 (36.90%) |
|
Tumor Size (mean ± SD, cm) |
3.33 ± 0.73 |
2.93 ± 0.72 |
3.86 ± 0.24 |
|
Tumor stage I II III IV N/A |
310 (33.41%) 3 (0.32%) 106 (11.42%) 312 (33.62%) 197 (21.23%) |
310 (52.36%) 3 (0.51%) 47 (7.94%) 35 (5.91%) 197 (33.28%) |
0 (0.00%) 0 (0.00%) 59 (17.56%) 277 (82.44%) 0 (0.00%) |
|
Tumor type Adenocarcinoma Squamous cell carcinoma Adenosquamous carcinoma Bronchoalveolar carcinoma Large cell neuroendocrine carcinoma Large cell carcinoma N/A |
608 (65.52%) 181 (19.50%) 7 (0.75%) 8 (0.86%) 1 (0.11%) 12 (1.29%) 111 (11.97%) |
372 (62.84%) 143 (24.16%) 7 (1.18%) 8 (1.35%) 1 (0.17%) 12 (2.03%) 49 (8.27%) |
236 (70.24%) 38 (11.31%) 0 (0.00%) 0 (0.00%) 0 (0.00%) 0 (0.00%) 62 (18.45%) |
|
Location (Lobe) Upper and middle Lower N/A |
258 (27.80%) 143 (15.41%) 527 (56.79%) |
174 (29.39%) 106 (17.91%) 312 (52.70%) |
84 (25.00%) 37 (11.01%) 215 (63.99%) |
|
Management MWA MWA + Chemotherapy |
592 (63.79%) 336 (36.21%) |
592 (100%) 0 (0%) |
0 (0%) 336 (100%) |
|
MWA characteristics |
|
|
|
|
Guidance CT scan |
928 (100%) |
592 (100%) |
336 (100%) |
|
Frequency (MHz) 2450 N/A |
705 (75.97%) 223 (24.03%) |
436 (73.65%) 156 (26.35%) |
269 (80.06%) 67 (19.94%) |
|
Antenna length (Max, mm) 200 180 140 N/A |
46 (4.96%) 690 (74.35%) 15 (1.62%) 177 (19.07%) |
46 (7.77%) 421 (71.11%) 15 (2.53%) 110 (18.59%) |
0 (0.00%) 269 (80.06%) 0 (0.00%) 67 (19.94%) |
|
Antenna diameter (Max, G) 20 19 18 17 N/A |
390 (42.03%) 55 (5.93%) 233 (25.11%) 68 (7.33%) 182 (19.60%) |
121 (20.44%) 55 (9.29%) 233 (39.36%) 68 (11.49%) 115 (19.42%) |
269 (80.06%) 0 (0.00%) 0 (0.00%) 0 (0.00%) 67 (19.94%) |
|
Active tip (mm) 16 15 5 N/A |
15 (1.62%) 143 (15.41%) 19 (2.05%) 751 (80.92%) |
15 (2.53%) 143 (24.16%) 19 (3.21%) 415 (70.10%) |
0 (0.00%) 0 (0.00%) 0 (0.00%) 336 (100.00%) |
|
Anesthesia type Conscious sedation Local anesthesia Local anesthesia + Conscious sedation Local anesthesia + intravenous N/A |
109 (11.75%) 657 (70.80%) 15 (1.62%) 33 (3.56%) 114 (12.27%) |
109 (18.41%) 321 (54.22%) 15 (2.53%) 33 (5.57%) 114 (19.27%) |
0 (0.00%) 336 (100.00%) 0 (0.00%) 0 (0.00%) 0 (0.00%) |
|
Power (Mean, W), 110 100 80 70 60 40 N/A |
71.76 ± 21.86 15 (1.62%) 184 (19.83%) 279 (30.06%) 175 (18.86%) 54 (5.82%) 175 (18.86%) 46 (4.95%) |
64.55 ± 22.96 15 (2.53%) 0 (0.00%) 166 (28.04%) 136 (22.97%) 54 (9.12%) 175 (29.56%) 46 (7.78%) |
85.00 ± 12.25 0 (0.00%) 184 (54.76%) 113 (33.63%) 39 (11.61%) 0 (0.00%) 0 (0.00%) 0 (0.00%) |
|
Ablation time (mean ± SD, max, min) |
14.38 ± 7.50 |
11.86 ± 4.80 |
18.40 ± 9.76 |
|
Ablation beyond margin (max, mm) 10 5 N/A |
481 (51.83%) 187 (20.15%) 260 (28.02%) |
297 (50.17%) 102 (17.23%) 193 (32.60%) |
184 (54.76%) 85 (25.30%) 67 (19.94%) |
|
Chemotherapy drug (for cases with available data) Pemetrexed Docetaxel Paclitaxel Gemcitabine Tigio Cisplatin Carboplatin Nedaplatin |
139 (14.98%) 41 (4.42%) 17 (1.83%) 30 (3.23%) 3 (0.32%) 40 (4.31%) 19 (2.05%) 89 (9.59%) |
- - - - - - - - |
139 (41.37%) 41 (12.20%) 17 (5.06%) 30 (8.93%) 3 (0.89%) 40 (11.90%) 19 (5.65%) 89 (26.49%) |
|
Complications (total number) PTX PE Emphysema Hemoptysis Pneumonia Pulmonary hemorrhage Pain Fever Infection Pulmonary abscess |
461 (49.68%) 176 (18.97%) 76 (8.19%) 16 (1.72%) 48 (5.17%) 14 (1.51%) 21 (2.26%) 60 (6.47%) 22 (2.37%) 27 (2.91%) 1 (0.11%) |
351 (59.29%) 140 (23.65%) 54 (9.12%) 16 (2.70%) 31 (5.24%) 14 (2.36%) 21 (3.55%) 44 (7.43%) 19 (3.21%) 11 (1.86%) 1 (0.17%) |
110 (32.74%) 36 (10.71%) 22 (6.55%) 0 (0.00%) 17 (5.06%) 0 (0.00%) 0 (0.00%) 16 (4.76%) 3 (0.89%) 16 (4.76%) 0 (0.00%) |
|
Local progression YES NO N/A |
90 (9.70%) 324 (34.91%) 514 (55.39%) |
47 (7.94%) 134 (22.64%) 411 (69.42%) |
43 (12.80%) 190 (56.55%) 103 (30.65%) |
|
Local recurrence YES NO N/A |
87 (9.38%) 269 (28.99%) 572 (61.63%) |
87 (14.70%) 269 (45.44%) 236 (39.86%) |
0 (0.00%) 0 (0.00%) 336 (100%) |
|
Distant recurrence YES NO N/A |
245 (26.40%) 241 (25.97%) 442 (47.63%) |
230 (38.85%) 217 (36.66%) 145 (24.49%) |
15 (4.46%) 24 (7.14%) 297 (88.40%) |
|
Local recurrence + Distant recurrence YES NO N/A |
13 (1.40%) 137 (14.76%) 778 (83.84%) |
12 (2.03%) 99 (16.72%) 481 (81.25%) |
1 (0.30%) 38 (11.30%) 297 (88.40%) |
|
Complete response YES NO N/A |
276 (29.74%) 67 (7.22%) 585 (63.04%) |
70 (11.82%) 9 (1.52%) 513 (86.66%) |
206 (61.31%) 58 (17.26%) 72 (21.43%) |
|
Partial response YES NO N/A |
82 (8.84%) 139 (14.98%) 707 (76.18%) |
5 (0.84%) 28 (4.73%) 559 (94.43%) |
77 (22.92%) 111 (33.04%) 148 (44.04%) |
|
Median OS (range, months) |
21.30-69.60 |
24.90-69.60 |
21.30-23.90 |
|
Median PFS (range, months) |
3.60-14.10 |
11.00-14.10 |
3.60-10.90 |
|
Median follow-up time (range, months) |
6.00-55.20 |
12.00-55.20 |
6.00-21.00 |
|
SD: Standard deviation, N/A: Not available, MWA: Microwave ablation, CT: Computed tomography,MHz: Megahertz, Max: Maximum, W: Watt, cm: centimeter, mm: millimeter, G: Gauge, min: Minute, PTX: Pneumothorax, PE: Pleural effusion, OS: Overall survival, PFS: Progression-free survival. |
|||
MWA and Chemotherapy Characteristics
MWA was used as a standalone treatment in 592 cases (63.80%) and combination with chemotherapy in 336 cases (36.20%). Computed tomography scan was consistently utilized for imaging guidance in all cases. The MWA frequency was predominantly set at 2450 MHz in 705 cases (75.97%). The most commonly used antenna specifications included a maximum length of 180 mm in 690 cases (74.35%) and a gauge of 20 in 390 cases (42.03%). For cases where data were available, the active tip length of the antenna was primarily 15 mm (15.41%). MWA procedures were frequently performed under local anesthesia in 657 cases (70.80%). The mean power used was 71.76 ± 21.86 watts, with 80 watts being the most common setting in 279 cases (30.06%). The average duration for maximum ablation was 14.38 ± 7.50 minutes. Regarding the ablation zone, a maximum extension of 10 mm beyond the tumor margin was used in 481 cases (51.83%) and 5 mm in 187 cases (20.15%), while the margin was unknown in the remaining cases (28.02%). The chemotherapy regimen included pemetrexed (41.37%), docetaxel (12.20%), paclitaxel (5.06%), gemcitabine (8.93%), tigio (0.89%), cisplatin (11.90%), carboplatin (5.65%), and nedaplatin (26.49%) (Table 3).
Safety and Efficacy
Complications were more prevalent in patients who underwent MWA alone (59.29%) compared to those who received MWA combined with chemotherapy (32.74%). The most frequent complication was pneumothorax, occurring in 176 cases (18.97%), with 140 cases (23.65%) in the MWA alone group and 36 cases (10.71%) in the combination group. Among the complications, only infection was higher in the MWA combined with the chemotherapy group (4.76% vs. 1.86%). Patients with available data and treated with MWA alone experienced higher local progression (26% vs. 18.5%), distant recurrence (51.5% vs. 38.5%), and both local and distant recurrence (10.8% vs. 2.6%). Reported complete response was 88.6% among cases that underwent MWA alone. While it was 78.0% in those who underwent combined MWA and chemotherapy. The median OS was higher in cases that underwent MWA alone (24.9 to 69.6 months vs. 21.3 to 23.90 months). Despite that, a significant number of studies across both treatment groups did not provide comprehensive recurrence data. Specifically, ten studies, encompassing 572 cases (61.63%), did not report on local recurrence, while eleven studies, involving 442 cases (47.63%), lacked data on distant recurrence. Additionally, thirteen studies, including 778 cases (83.84%), failed to report on both local and distant recurrence. Furthermore, ten studies covering 585 cases (63.04%) omitted information on the complete response, and eleven studies involving 707 cases (76.18%) did not document partial response (Table 3).
Discussion
It has been indicated that NSCLC tumors develop through progressive pathological changes and exhibit a few unique molecular signatures of genomic alterations. The alterations typically occur in the cells lining the airways, which are predominantly exposed to harmful chemicals such as tobacco smoke carcinogens and environmental pollutants like asbestos, nickel, and arsenic. As these precancerous cells progress through various stages of tumorigenesis, they undergo changes, including hyperplasia, squamous metaplasia, squamous dysplasia, and eventually carcinoma in situ [3].
Xu et al. conducted a cohort study involving 319 patients undergoing MWA for NSCLC management, reported a mean age of 68.0 ± 10.6 years, with a male predominance (61.4%) [25]. Similarly, the present study found a mean age of 69.52 ± 9.07 years, with a similar male representation (62.50%) in the MWA alone group. In an RCT by Wei et al., which examined the use of MWA combined with chemotherapy for NSCLC management, the mean age of patients was 59 years, with a male predominance (65%) [22]. However, the mean age of cases who underwent MWA combined with chemotherapy in the present study was 62.28 ± 6.96 years, with a male ratio of 63.10%. The group of MWA alone had a smaller mean tumor size of 2.93 ± 0.72 cm compared to a study by Yao et al., which reported a mean tumor size of 3.01 ± 1.11 cm [19]. Conversely, the group of MWA combined with chemotherapy had a larger mean tumor size of 3.86 ± 0.24 cm, which was greater than the 3.6 cm reported in another study [22].
Primarily, there are three types of NSCLC: adenocarcinoma, which accounts for 40% of cases; squamous cell carcinoma (25-30%) and large cell carcinoma (5-10%) [26]. In this systematic review, adenocarcinoma was also the predominant subtype, but at a higher percentage of 65.52%. Squamous cell carcinoma was found in 19.50%, consistent with expected patterns, while large cell carcinoma was identified in only 1.29%, significantly lower than that reported. Lv et al. reported that, among 118 cases, the most common tumor locations were the upper and middle lobes of the lung, observed in 73 patients (61.86%), while 45 patients (38.14%) had the tumor in the lower lobe [12]. In the present study, in 258 cases (27.80%), the tumors were located in the upper and middle lobes, while in 143 cases (15.41%), the lower lobe was involved. However, a substantial proportion of the studies (56.79%) had missing data regarding tumor location.
MWA has emerged as a promising option for treating NSCLC, particularly in cases where surgery is not feasible. Since its introduction in 2002, MWA has gained recognition as a viable alternative for early-stage NSCLC [8]. Wu et al. reported that percutaneous image-guided ablation, including MWA, offers comparable OS rates to stereotactic ablative radiotherapy for inoperable early-stage primary lung cancer. Additionally, propensity-score-weighted analyses have demonstrated that MWA achieves OS and disease-free survival rates similar to surgery [8]. Li et al. highlighted the advantages of MWA's minimally invasive nature, making it a favorable choice for preserving pulmonary function. This treatment is particularly suitable for stage I NSCLC patients who are medically inoperable due to high-risk conditions or who prefer not to undergo surgery [14]. Shan et al. also emphasized MWA's efficacy in effectively destroying tumor cells, with some patients achieving outcomes comparable to surgery. This reduction in tumor burden improves tumor-free survival and enhances patients' quality of life and mental well-being [7]. Furthermore, Huang et al. underscored the potential of MWA not only for inoperable early-stage NSCLC but also as a palliative option for advanced NSCLC when combined with systemic chemotherapy. This combination could significantly extend both progression-free survival (PFS) and OS [27]. These studies highlight MWA as an effective and versatile treatment option across different stages of NSCLC. In this systematic review, 52.87% of the patients in the MWA alone group had early-stage NSCLC, while 13.85% were diagnosed with advanced disease. In contrast, the disease in all patients (100%) in MWA combined with the chemotherapy group was advanced.
A study reported a maximum ablation time of 8 minutes [15], while another one utilized a maximum ablation time of 20 minutes [9]. In contrast, this systematic review showed the mean maximum ablation time of 11.86 ± 4.80 minutes in the MWA alone group. This indicates that the ablation times reported in individual studies fell within the broader range observed in the systematic review, exhibiting some variation but generally consistent with the overall average. Wei et al. conducted an RCT on patients who received MWA combined with chemotherapy, using a maximum ablation time of 8 minutes [22]. Conversely, another study reported a maximum ablation time of 33 minutes [17]. In the present study, the mean maximum ablation time for cases who underwent MWA combined with chemotherapy was 18.40 ± 9.76 minutes. This indicates a notable increase in ablation time when MWA is combined with chemotherapy.
Regarding safety, Xu et al., among 391 cases of NSCLC underwent MWA, reported a complication rate of 60.50%, with pneumothorax being the most common complication (49.22%), followed by emphysema (42.49%) [25]. Similarly, the complication rate among cases who underwent only MWA in the current study was 59.29%, higher than the other group (32.74%), with pneumothorax being the most common complication (23.65%), followed by pleural effusion (9.12%). A study conducted in 2019 reported a local progression rate of 26.79% after MWA treatment [16]. In contrast, another study found a significantly lower incidence of local progression of 8.82% [28]. In this study, among the cases of the MWA alone group with available data, the incidence of local progression was 25.97%. In patients treated with MWA combined with chemotherapy, one study reported a local progression rate of 18.24% [22]. Another study found a similar local progression rate of 17.95% [24]. In this study, the local progression rate in combined regimens was 18.45% for available case data, closely aligning with the reported incidence rates [11,20]. In the current study, local recurrence occurred in 87 cases out of 356 (24.44%) with available data in the MWA alone group. Meanwhile, Wei et al. A local recurrence rate of 6.33% was reported, and Yang et al. observed a higher rate of 27.66% [20,29]. The reviewed studies on MWA combined with chemotherapy did not provide any data on local recurrence, highlighting a significant gap in the reported outcomes for this group.
Within the reviewed cases with available data who underwent MWA alone, the treatment resulted in a complete response rate of 88.6% (70 out of 79 cases) and a partial response rate of 15.2 % (5 out of 33 cases). Wei Z et al. reported a slightly higher complete response rate of 91.14% with a lower partial response rate of 8.86% [29]. Another study found a complete response rate of 75% and a partial response rate of 25% with the same treatment modality [28]. In the group of MWA combined with chemotherapy of this study, the complete and partial response rates were 78.0% and 41.0%, respectively. However, the high percentage of cases with unknown data regarding response rate should not be overlooked. Shan et al. reported a complete response rate of 23.53% and a partial response rate of 47.06% [7]. In contrast, Wei et al. observed a higher complete response rate of 84.78% and a lower partial response rate of 15.22% [23].
Xu et al. reported a median OS of 17.0 ± 10.9 months and a median PFS of 13.0 ± 10.5 months in patients treated with MWA [25]. Additionally, another study documented a median OS of 20 months [30]. The studies included in the group of MWA alone of this systematic review reported a broader range of outcomes, with median OS varying from 24.90 to 69.60 months and median PFS ranging from 11.00 to 14.10 months. The median OS for that group was generally higher than that reported by Xu et al. and Pusceddu et al. [25,30]. In addition, a study by Huang et al. reported a median OS of 18.8 months and a median PFS of 8.1 months for patients treated with MWA combined with chemotherapy [27]. The patients with the combined regimen in the present study showed slightly higher median OS values, ranging from 21.30 to 23.90 months, but a broader range of median PFS, from 3.60 to 10.90 months.
This study was constrained by the significant absence of critical outcome data in many studies, including local and distant recurrence rates, treatment responses, and survival rates. Specifically, the lack of local and distant recurrence data in 61.63% and 47.63% of cases, respectively, impairs a comprehensive assessment of the efficacy of MWA alone or in combination with chemotherapy.
Conclusion
MWA combined with chemotherapy may represent a more effective option, with a slightly similar treatment response, reducing the risk of recurrence and minimizing complications. However, the influence of the tumor stage on outcomes may not be excluded.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: MGHH, BAA, AMA, and YMM were responsible for the manuscript's data collection, analysis, and final approval. FHK and AMS were significant contributors to the conception of the study, as well as to the literature search for related studies. RMA, SHT, AMS, LRAP, and AJQ were involved in the literature review, the design of the study, and the critical revision of the manuscript. SHM, HOA, HKA, and FA were involved in the literature review, the writing of the manuscript, and the design of the study and interpretation. BAA and FHK confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
References
- Duma N, Santana-Davila R, Molina JR. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. InMayo Clinic Proceedings 2019;94(8):1623-40. doi:10.1016/j.mayocp.2019.01.013
- Mingomataj E, Krasniqi M, Dedushi K, Sergeevich KA, Kust D, Qadir AA, et al. Cancer publications in one year (2023): a cross-sectional study. Barw Medical Journal. 2024;1(2):3-11. doi:10.58742/g9q9t715
- Kumar M, Sarkar A. Current therapeutic strategies and challenges in NSCLC treatment: A comprehensive review. Experimental oncology. 2022;44(1):7-16. doi:10.32471/exp-oncology.2312-8852.vol-44-no-1.17411
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. InMayo clinic proceedings. 2008; 83 (5): 584-94. doi:10.4065/83.5.584
- Rodak O, Peris-Díaz MD, Olbromski M, et al. Current landscape of non-small cell lung cancer: epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers. 2021;13(18):4705. doi:10.3390/cancers13184705
- Ali RM, Omar SS, Ahmed HK, Omar DA, Mahmood YM, Mustafa MQ, et al. Effect of Sunitinib in the Management of Lung Cancer: A Systematic Review of Clinical Trials. Barw Medical Journal. 2024; 2(2):57-64. doi:10.58742/bmj.v2i2.95.
- Shan Y, Yin X, Lin F, Wang C, Kong Y, Yao W. Chemotherapy combined with intermittent microwave ablation in the treatment of oligometastatic non-small cell lung cancer. J BUON. 2021;26(2):320-7. doi:N/A
- Wu C, Cao B, He G, Li Y, Wang W. Stereotactic ablative brachytherapy versus percutaneous microwave ablation for early-stage non-small cell lung cancer: a multicenter retrospective study. BMC cancer. 2024;24(1):304. doi:10.1186/s12885-024-12055-6
- Han X, Yang X, Huang G, Li C, Zhang L, Qiao Y, et al. Safety and clinical outcomes of computed tomography‐guided percutaneous microwave ablation in patients aged 80 years and older with early‐stage non‐small cell lung cancer: A multicenter retrospective study. Thoracic cancer. 2019;10(12):2236-42. doi:10.1111/1759-7714.13209
- Ali RM, Omar SS, Kakamad FH, Omar DA, Mahmood YM, Mustafa MQ, et al. Efficacy of Sorafenib in the Management of Non-Small Cell Lung Cancer: A Systematic Review. Barw Medical Journal. 2024; 2(2):31-38. doi:10.58742/bmj.v2i2.92
- Abdullah HO, Abdalla BA, Kakamad FH, Ahmed JO, Baba HO, Hassan MN, et al. Predatory Publishing Lists: A Review on the Ongoing Battle Against Fraudulent Actions. Barw Medical Journal. 2024; 2(2): 26-30. doi:10.58742/bmj.v2i2.91
- Lv Y, Liu Y, Li K, Liu Z, Zhang T, Duan M, et al. Nomogram Based on Preoperative Absolute Lymphocyte Count to Predict Local Recurrence in Patients with Non-Small Cell Lung Cancer After Microwave Ablation. Journal of Inflammation Research. 2023:1761-70. doi:10.2147/JIR.S402108
- Xu S, He L, Qi J, Kong FL, Bie ZX, Li YM, et al. Percutaneous core-needle biopsy before and immediately after coaxial microwave ablation in solid non-small cell lung cancer: the comparison of genomic testing from specimens. Cancer Imaging. 2023;23(1):93. doi:10.1186/s40644-023-00610-6.
- Li B, Bie Z, Li Y, Guo R, Wang C, Li X. Synchronous percutaneous core-needle biopsy and microwave ablation for stage I non-small cell lung cancer in patients with Idiopathic pulmonary fibrosis: initial experience. International Journal of Hyperthermia. 2023;40(1):2270793. doi:10.1080/02656736.2023.2270793.
- Hu H, Zhai B, Liu R, Chi JC. Microwave ablation versus wedge resection for stage I non-small cell lung cancer adjacent to the pericardium: propensity score analyses of long-term outcomes. Cardiovascular and Interventional Radiology. 2021; 44:237-46. doi:10.1007/s00270-020-02601-7.
- Das SK, Huang YY, Li B, Yu XX, Xiao RH, Yang HF. Comparing cryoablation and microwave ablation for the treatment of patients with stage IIIB/IV non-small cell lung cancer. Oncology letters. 2020;19(1):1031-41. doi:10.3892/ol.2019.11149.
- Wei Z, Li Q, Ye X, Yang X, Huang G, Li W, et al. Microwave ablation or plus monochemotherapy in elderly advanced non-small-cell lung cancer patients. Minimally Invasive Therapy & Allied Technologies. 2021;30(2):106-14. doi:10.1080/13645706.2019.1678173.
- Wang Y, Liu B, Cao P, Wang W, Wang W, Chang H, et al. Comparison between computed tomography‐guided percutaneous microwave ablation and thoracoscopic lobectomy for stage I non‐small cell lung cancer. Thoracic cancer. 2018;9(11):1376-82. doi:10.1111/1759-7714.12842.
- Yao W, Lu M, Fan W, Huang J, Gu Y, Gao F, et al. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. International Journal of Hyperthermia. 2018;34(8):1329-36. doi:10.1080/02656736.2018.1434901.
- Yang X, Ye X, Zheng A, Huang G, Ni X, Wang J, et al. Percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: Clinical evaluation of 47 cases. Journal of surgical oncology. 2014;110(6):758-63. doi:10.1002/jso.23701
- Liu H, Steinke K. High‐powered percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: a preliminary study. Journal of medical imaging and radiation oncology. 2013;57(4):466-74. doi:10.1111/1754-9485.12068
- Wei Z, Yang X, Ye X, Feng Q, Xu Y, Zhang L, et al. Microwave ablation plus chemotherapy versus chemotherapy in advanced non-small cell lung cancer: a multicenter, randomized, controlled, phase III clinical trial. European radiology. 2020; 30:2692-702. doi:10.1007/s00330-019-06613-x
- Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Medical Oncology. 2015; 32:1-8. doi:10.1007/s12032-014-0464-z
- Wei Z, Ye X, Yang X, Zheng A, Huang G, Li W, et al. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovascular and interventional radiology. 2015; 38:135-42. doi:10.1007/s00270-014-0895-0.
- Xu S, Bie ZX, Li YM, Li B, Guo RQ, Li XG. Computed tomography‐guided microwave ablation for the treatment of non‐small cell lung cancer patients with and without adjacent lobe invasion: A comparative study. Thoracic Cancer. 2021;12(20):2780-8. doi:10.1111/1759-7714.14125.
- Ald Alduais Y, Zhang H, Fan F, Chen J, Chen B. Non-small cell lung cancer (NSCLC): a review of risk factors, diagnosis, and treatment. Medicine. 2023;102(8). doi:10.1097/MD.0000000000032899
- Huang G, Li W, Meng M, Ni Y, Han X, Wang J, et al. Synchronous microwave ablation combined with cisplatin intratumoral chemotherapy for large non-small cell lung cancer. Frontiers in Oncology. 2022; 12:955545. doi:10.3389/fonc.2022.955545
- Wei Z, Wang Q, Ye X, Yang X, Huang G, Li W, et al. Microwave ablation followed by immediate biopsy in the treatment of non-small cell lung cancer. International Journal of Hyperthermia. 2018;35(1):262-8. doi:10.1080/02656736.2018.1494856
- Wei Z, Ye X, Yang X, Huang G, Li W, Han X, et al. Efficacy and safety of microwave ablation in the treatment of patients with oligometastatic non-small-cell lung cancer: a retrospective study. International Journal of Hyperthermia. 2019;36(1):826-33. doi:10.1080/02656736.2019.1642522
- Pusceddu C, Melis L, Sotgia B, Guerzoni D, Porcu A, Fancellu A. Usefulness of percutaneous microwave ablation for large non small cell lung cancer: A preliminary report. Oncology Letters. 2019;18(1):659-66. doi:10.3892/ol.2019.10375

This work is licensed under a Creative Commons Attribution 4.0 International License.

