Edoxaban and Cancer-Associated Venous Thromboembolism: A Meta-analysis of Clinical Trials
Abstract
Introduction
Cancer patients face a venous thromboembolism (VTE) risk that is up to 50 times higher compared to individuals without cancer. In 2010, direct oral anticoagulants (DOACs) such as apixaban, rivaroxaban, and edoxaban were introduced, consequently becoming the controversial oral anticoagulants for VTE therapy. This study is a meta-analysis of randomized clinical trials (RCTs) evaluating the use of edoxaban for treating VTE in cancer patients over different treatment durations.
Methods
Using Google Scholar, a systematic search for RCTs on edoxaban for cancer-associated VTE was performed. The data extracted covered patient numbers, age, gender, BMI, cancer type, edoxaban dosage, treatment duration, comorbidities, major bleeding, recurrent VTE incidence, and deaths. Statistical significance was set at 0.05.
Results
Out of 52 studies, nine with 3,190 cases met the inclusion criteria. The mean age was 66.68 years, with 1,604 females (50.28%). Major bleeding occurred in 192 patients (7.66%) in the 6- or 12-month group and 57 (8.35%) in the 3-month group (p=0.573). Recurrent VTE was observed in 145 patients (5.78%) in the 6- or 12-month group and 95 (13.91%) in the 3-month group (p<0.001). Deaths from any cause totaled 548 (21.86%) in the 6- or 12-month group and 165 (24.16%) in the 3-month group (p=0.110).
Conclusion
Cancer patients receiving edoxaban for six or 12 months experience a lower recurrence rate of VTE compared to those on a 3-month treatment. The incidence of major bleeding appears to be similar between the two treatment durations.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), frequently occurs in cancer patients. Cancer is a condition characterized by abnormal cells' uncontrolled proliferation and persistence. Those with VTE who have cancer are at a higher risk of experiencing recurrent thromboembolism [1, 2].
Patients with cancer face a risk of VTE that is up to 50 times greater compared to those without cancer. The annual incidence of VTE in cancer patients varies between 3% and 15% [3]. Risk factors for developing cancer-associated thrombosis (CAT) extend beyond large pelvic masses that compress the iliac veins. They also include comorbidities, immobilization, chemotherapy, targeted therapies (such as bevacizumab), surgeries like lymphadenectomy, and the presence of intravenous catheters. These factors can contribute to a prothrombotic or hypercoagulable state [4].
The VTE is linked with considerable morbidity and mortality. Therefore, it is crucial to start anticoagulant therapy promptly and to maintain it for as long as the patient remains at elevated risk for recurrent events [5]. Anticoagulant therapy poses challenges in cancer patients due to their increased risk of both recurrent VTE and major bleeding compared to those without cancer. These complications can interfere with cancer treatments [5].
Low-molecular-weight heparin (LMWH), unfractionated heparin (UFH), and vitamin K antagonists (VKA) have been utilized in the treatment of CAT. Major guidelines, including those from the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Thoracic Society, recommend LMWH for treating CAT [4]. Nonetheless, the effectiveness of LMWH beyond six months is not well established, and its use can be challenging due to the need for daily subcutaneous injections [4].
In 2010, direct oral anticoagulants (DOACs) like apixaban, rivaroxaban, and edoxaban were introduced. These DOACs, which include factor Xa and thrombin inhibitors, have minimal interactions with food or other medications, like LMWH. This property enables fixed dosing without the need for regular coagulation monitoring [1, 4].
Recent large randomized controlled trials (RCTs) have demonstrated the benefits of DOACs over conventional VKAs for treating VTE. Consequently, DOACs have become the preferred oral anticoagulant for VTE therapy [6,7]. Moreover, DOACs have been shown to be non-inferior to LMWH when treating CAT. As a result, the use of DOACs in CAT patients is on the rise [8,9].
The optimal duration of anticoagulation therapy is determined by weighing the risk of recurrent VTE if the treatment is discontinued against the risk of bleeding if it is continued. Factors such as patient preference, life expectancy, and cost should also be considered [5].
This study is a meta-analysis of RCTs assessing the use of edoxaban for VTE in cancer patients across various treatment durations.
Methods
Study design
This meta-analysis was conducted strictly following the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Data sources and search strategy
A meta-analysis was conducted using Google Scholar to identify all published randomized studies on edoxaban treatment for cancer-associated VTE. The search used the following keywords: (Edoxaban OR Lixiana OR cancer OR tumor OR malignancy OR malignant OR cancerous OR tumorous AND thromboembolism OR embolism OR thrombosis).
Eligibility criteria
In this meta-analysis, only RCT studies were included. Studies were excluded if they were 1) non-English, 2) only abstracts, or 3) did not meet the inclusion criteria. All references included in this study were assessed for eligibility [10].
Study selection and data extraction
The titles and abstracts of the identified studies were first screened, followed by a thorough full-text screening to determine eligibility. Various data were extracted from the included studies, including number of patients, age, gender, BMI, type of cancer, edoxaban dosage, treatment duration, comorbidities, history of major bleeding and VTE, incidence of major bleeding and recurrent VTE during Edoxaban treatment, and the number of deaths.
Statistical analyses
The data were first used in a qualitative synthesis and then quantitatively re-analyzed using the Chi-square test and Fisher's exact test with the Statistical Package for Social Sciences (SPSS) version 27.0. The level of statistical significance was set at 0.05.
Results
A total of 52 studies were found in the search. Sixteen were directly excluded due to duplication, non-English language, or being pre-prints. The remaining 36 studies were screened by reviewing their titles and abstracts, with none removed after screening. All 36 studies underwent full-text screening, where 27 were excluded for various reasons. The remaining studies were evaluated for eligibility (Fig. 1); ultimately, nine studies [3-5, 8, 11-15], comprising 3,190 cases, met the inclusion criteria (Table 1). The mean age of the patients was 66.68 ± 10.70 years, with a predominance of females, accounting for 1,604 (50.28%) of the total population. Among the comorbidities, hypertension was the most common, affecting 535 patients (16.77%), followed by diabetes in 208 patients (6.52%). A history of major bleeding or recurrent VTE was reported in 210 patients (6.58%). Gastrointestinal tract cancer was the most frequently mentioned type of cancer in the included studies, affecting 273 patients (8.56%).
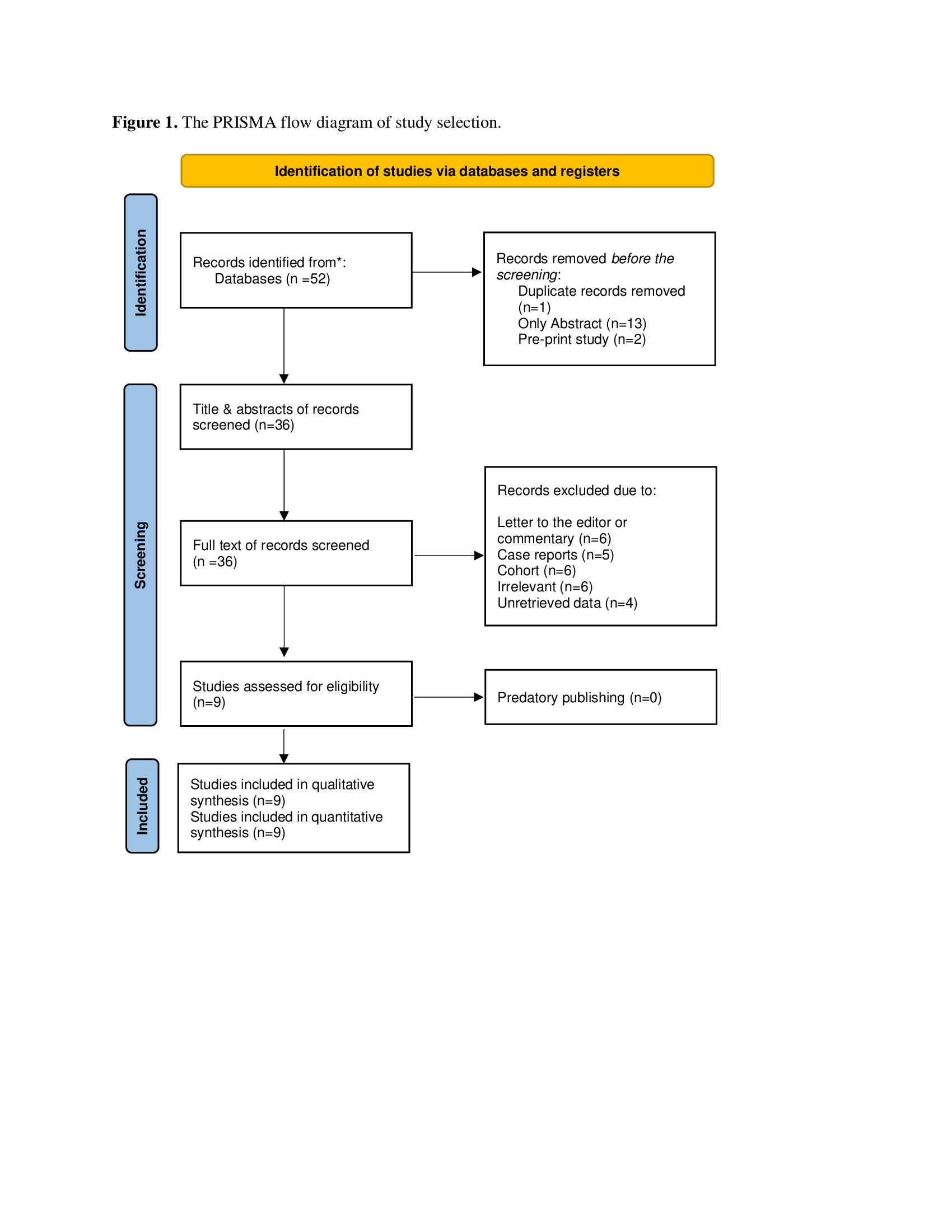
|
Author, year (Reference) |
Number of cases |
Study design |
Age (mean, SD) |
Gender (M/F) |
BMI (mean, SD) |
Dose |
Treatment period |
Outcome |
||||
|
60 mg |
30 mg |
3 mo |
≥ 6 mo |
Major Bleed |
Rec. VTE |
All causes Death |
||||||
|
Chatani et al. 2024 (11) |
151 |
RCT |
67.6 ± 9.8 |
81/70 |
26.7 ± 4.0 |
151 |
0 |
71 |
80 |
14 |
29 |
29 |
|
450 |
71.9 ± 9.7 |
86/364 |
21.1 ± 3.0 |
0 |
450 |
234 |
216 |
36 |
65 |
114 |
||
|
Chung et al. 2024 (12) |
20 |
RCT |
66.2±12.3 |
12/8 |
21.4±2.7 |
20 |
0 |
20 |
0 |
9 |
2 |
3 |
|
Di Nisio et al. 2019 (5) |
294 |
RCT |
64.4±11.1 |
156/ 138 |
N/A |
240 |
54 |
0 |
294 |
5 |
2 |
39 |
|
Mulder et al. 2020 (3) |
477 |
RCT |
N/A |
N/A |
N/A |
263 |
214 |
0 |
477 |
29 |
36 |
0 |
|
Nakamura et al. 2022 (13) |
53 |
RCT |
64.48±9.93 |
28/ 25 |
N/A |
21 |
31 |
53 |
0 |
4 |
3 |
3 |
|
Oride et al. 2023 (4) |
16 |
RCT |
60.2±10.5 |
0/16 |
22.1±3.90 |
3 |
13 |
0 |
16 |
1 |
2 |
0 |
|
Raskob et al. 2016 (14) |
378 |
RCT |
66.0 ± 13.0 |
181/197 |
N/A |
281 |
97 |
0 |
378 |
47 |
14 |
40 |
|
Raskob et al. 2018 (8) |
522 |
RCT |
64.3±11.0 |
277/ 245 |
N/A |
522 |
0 |
0 |
522 |
36 |
41 |
206 |
|
Yamashita et al. 2023 (15) |
296 |
RCT |
71.6±9.4 |
94/202 |
22.7±4.0 |
80 |
216 |
0 |
296 |
28 |
3 |
66 |
|
305 |
70.1±10.3 |
73/ 232 |
22.4±4.1 |
71 |
234 |
305 |
0 |
22 |
22 |
77 |
||
| RCT: randomized clinical trial, SD: standard deviation, M: male; F: Female, mg: milligram, mo: month, Rec: recurrent, VTE: venous thromboembolism, N/A: not applicable | ||||||||||||
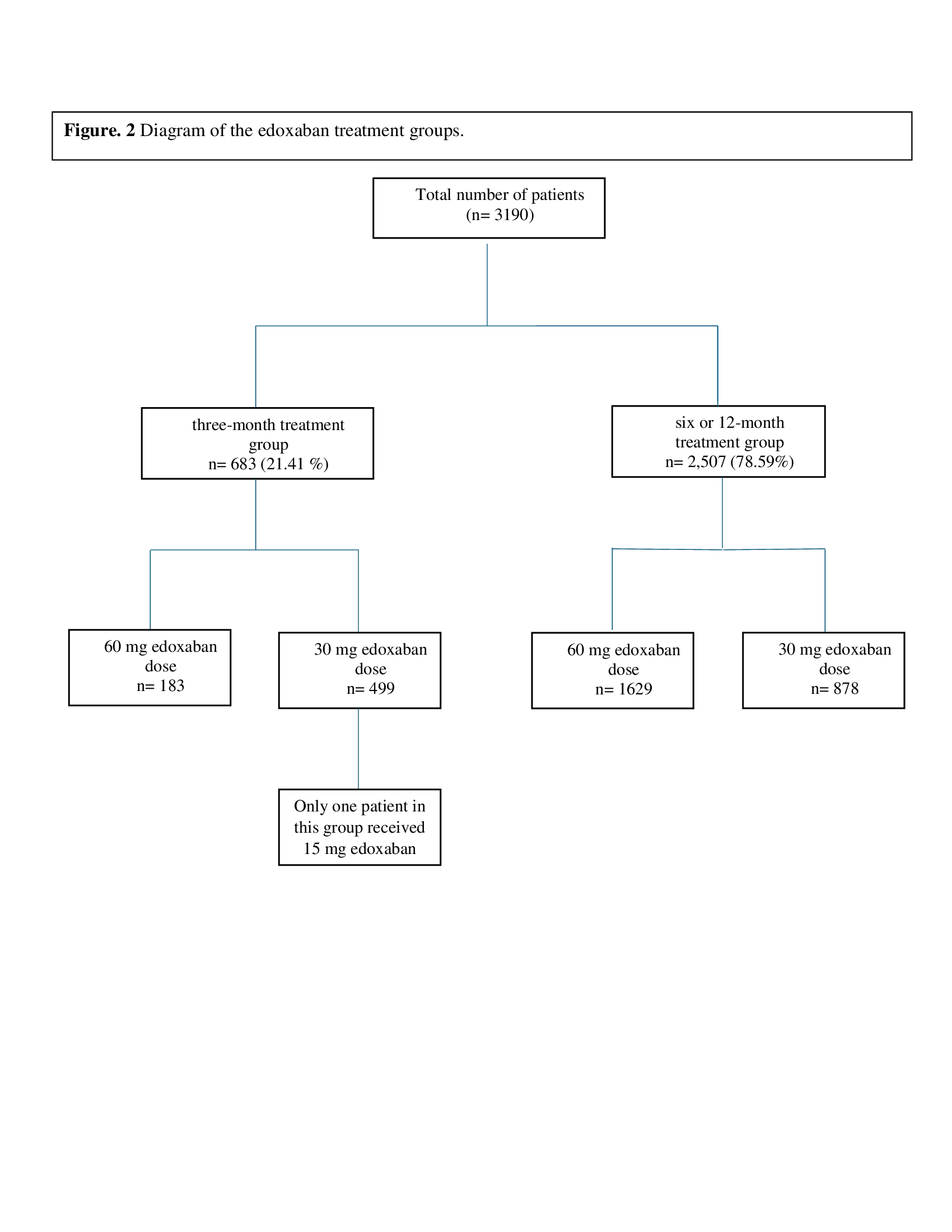
Regarding Edoxaban dosage, the standard dose of 60 mg/day was administered to 1,812 patients (56.80%), while a reduced dose of 30 mg/day was given to 1,377 patients (43.17%). Only one patient received a 15 mg/day dose. The studies included in the review used three different treatment durations: 3 months, 6 months, or 12 months. A total of 2,507 patients, 78.59%, received edoxaban for either a 6-month or 12-month period, and 683 patients (21.41 %) received edoxaban for three months (Fig. 2). Major bleeding occurred in 192 patients (7.66 %) in the 6 or 12-month group and 57 patients (8.35%) in the 3-month group (p-value=0.573) (Table 2).
|
Variables |
Frequency/ percentages |
|
Number of total patients Age (mean of means) ± SD BMI (mean of means) ± SD a Body weight ≤60 kg, n (%) b |
3,190 (100%) 66.68 ± 10.70 22.73 ± 3.67 1,041 (32.63%) |
|
Gender, n (%) |
|
|
Female Male N/A |
1,604 (50.28 %) 1,109 (34.77%) 477 (14.95%) |
|
Hypertension, n (%) Yes No Unknown |
535 (16.77 %) 687 (21.54 %) 1,968 (61.69%) |
|
Diabetes mellitus, n (%) Yes No Unknown |
208 (6.52%) 1,014 (31.79%) 1,968 (61.69%) |
|
History of VTE, n (%) Yes No Unknown |
164 (5.14%) 2,082 (65.27%) 944 (29.59%) |
|
History of major bleeding, n (%) Yes No Unknown |
46 (1.44%) 1,156 (36.24%) 1,988 (62.32%) |
|
History of stroke, n (%) Yes No Unknown |
55 (1.72%) 1,167 (36.58%) 1,968(61.70%) |
|
Type of cancer, n (%) Gastrointestinal tract cancer Breast cancer Lung cancer Gynecological cancer Urogenital cancer Hematological malignancy Other known malignancies Unknown |
273 (8.56%) 134 (4.20%) 122 (3.83%) 105 (3.29%) 92 (2.88%) 79 (2.48%) 150 (4.70%) 2,235 (70.06%) |
|
Overall edoxaban dose group, n (%) 60 mg 30 mg 15 mg |
1,812 (56.80%) 1,377 (43.17%) 1 (0.03%) |
|
Treatment period, n (%) six or 12-month treatment 60 mg 30 mg |
2,507 (78.59%) 1629 (64.98%) 878 (35.02%) |
|
three-month treatment 60 mg 30 mg 15 mg |
683 (21.41 %) 183 (26.79%) 499 (73.06%) 1 (0.15%) |
|
Major bleeding during the treatment, n (%) six or 12-month treatment three-month treatment Overall |
192 (7.66%) 57 (8.35%) 249 (7.80%) |
|
Recurrence VTE, n (%) six or 12-month treatment three-month treatment Overall |
145 (5.78%) 95 (13.91%) 240 (7.52%) |
|
Number of deaths from all causes, n (%) six or 12-month treatment three-month treatment Overall |
548 (21.86%) 165 (24.16%) 713 (22.35%) |
|
a: The BMI belongs to 1,238 cases as it was provided in three studies, b: body weight ≤60 kg belongs to five of the included studies, HTN: hypertension, DM: diabetes mellitus, VTE: venous thromboembolism. |
|
Recurrent VTE was observed in 145 patients (5.78%) in the 6-month or 12-month group and 95 patients (13.91%) in the 3-month group (p-value<0.001). The number of deaths from any causes was 548 deaths (21.86%) in the 6-month or 12-month, followed by 165 deaths (24.16%) in the 3-month group (p-value=0.110) (Table 3).
|
Parameters |
6 or 12-month treatment |
3-month group |
p-value |
|
Major bleeding |
192 (7.66%) 2,315 (92.34) |
57 (8.35%) 626 (91.65) |
0.573 |
|
Recurrent VTE Yes No |
145 (5.78%) 2,362 (94.22%) |
95 (13.90%) 588 (86.10) |
<0.001 |
|
All causes of death Yes No |
548 (21.86%) 1,959 (78.14%) |
165 (24.16%) 518 (75.84%) |
0.110 |
Discussion
Thrombogenesis can be triggered by several factors, including age, a sedentary lifestyle, and advances in imaging technologies that have led to increased diagnosis rates. Other contributing factors include using hematopoietic agents, blood transfusions, invasive intravascular catheters, and the effects of new antineoplastic drugs [16].
The high incidence and recurrence of VTE in cancer patients is well-documented. The pathogenesis of cancer-associated coagulopathy is complex and involves several mechanisms [17]. Tumor cells can trigger blood coagulation through various processes, including producing procoagulant factors, fibrinolytic activities, and pro-aggregating effects [17]. The hemostatic side effects of oncological treatments, such as cytotoxic drugs, hormone therapy, anti-angiogenic therapy, radiotherapy, and surgery, also contribute to blood coagulopathies [18].
Treating VTE associated with active malignancy involves not only managing the thrombosis but also addressing the cancer itself. This approach is complicated by issues such as bleeding risks and the potential for recurrent VTE [19].
The LMWH has been the preferred anticoagulant for CAT following evidence from major RCTs that demonstrated its superiority over traditional vitamin K antagonists (VKAs) (20-23). Recently, several large RCTs have compared DOACs and LMWH, demonstrating that DOACs are non-inferior to LMWH for treating venous VTE in patients with CAT, and they have emerged as an alternative to LMWH [6,8,9].
Based on the results from several clinical trials, including Hokusai VTE Cancer (edoxaban vs. dalteparin, n=522 vs. 524), Caravaggio (apixaban vs. dalteparin, n=576 vs. 579), and SELECT-D (rivaroxaban vs. dalteparin, n=203 vs. 203), as well as meta-analyses incorporating these studies, DOACs are deemed effective, safe, and useful for treating CAT [6,8,9]. These trials indicated that the VTE recurrence rates with DOACs range from 4% to 8%, and the major bleeding rates range from 3% to 7%, which are comparable to those observed with LMWH (3). In line with the studies referenced, the present review found that the overall recurrence rate and major bleeding were 7.52% and 7.80%, respectively.
In a sub-analysis of the HOKUSAI trial, which included 771 cancer patients with VTE, edoxaban was associated with a trend toward lower VTE recurrence and significantly reduced clinically relevant bleeding compared to warfarin [14]. Similarly, other Xa inhibitors, such as rivaroxaban and apixaban, demonstrated comparable benefits in sub-analyses of the EINSTEIN-DVT and PE trials [24] and the AMPLIFY trial [6]. These findings suggest that Xa inhibitors may be as effective as, and potentially safer than, warfarin for treating VTE in cancer patients [17].
A recent RCT, the ONCO DVT study, demonstrated the potential benefits of extended anticoagulation therapy with edoxaban for patients with cancer-associated isolated distal DVT, particularly in reducing thrombotic risk [15]. However, concerns have been raised about the increased risk of bleeding associated with prolonged anticoagulation in patients with cancer-associated VTE. Therefore, individual risk assessment and tailored treatment strategies are crucial for optimizing management in these patients [11]. In their RCT, Chatani et al. found that the 12-month cumulative incidence of major bleeding was higher in the 12-month treatment group compared to the 3-month group for patients receiving 60 mg of edoxaban (14.3% vs. 4.4%, p-value = 0.046). However, for the 30 mg edoxaban subgroup, the incidence of major bleeding did not differ significantly between the two treatment durations (8.7% vs. 8.6%, p-value = 0.89). However, the cumulative 12-month incidence of symptomatic recurrent VTE was lower in the 12-month group compared to the 3-month group for both the 60 mg and 30 mg doses (1.1% vs. 7.6%, p-value = 0.002) [11]. In the current review, major bleeding occurred in 7.66% of patients in the 6 or 12-month treatment group and 8.35% of those in the 3-month group for both the 60 mg and 30 mg doses, with no statistically significant difference found (p-value = 0.573). Consistent with Chatani et al.’s findings, the recurrence rate of VTE was higher in the 3-month group for both doses.
In a RCT conducted by Raskob and colleagues, edoxaban was used for 522 cases of cancer-associated VTEs; the number of deaths from any cause in their study was 39.5% [8]. In the present review, the mortality rate from any cause was 22.35%. The comparison between the two groups for both the 60 mg and 30 mg doses showed no significant difference (p-value = 0.110).
Conclusion
Cancer patients receiving edoxaban for six or 12 months experience a lower recurrence rate of VTE compared to those on a 3-month treatment. The incidence of major bleeding appears to be similar between the two treatment durations.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: FHK, SSO and RMA were significant contributors to the conception of the study and the literature search for related studies. FJA, DHMS, MNH and KFH were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. HAN, NSS and MQM were involved in the literature review, study design, and manuscript writing. DAO, SHT, RJR, SHM and TNM Literature review, final approval of the manuscript, and processing of the tables. FHK and HAN confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
References
- Van Es N, Di Nisio M, Bleker SM, Segers A, Mercuri MF, Schwocho et al. Edoxaban for treatment of venous thromboembolism in patients with cancer. Thrombosis and haemostasis. 2015;114(12):1268-76. doi:10.1160/TH15-06-0452
- Mingomataj E, Krasniqi M, Dedushi K, Sergeevich KA, Kust D, Qadir AA. Cancer Publications in One Year (2023): A Cross-Sectional Study. Barw Medical Journal. 2024;2(1):3-11. doi:10.58742/g9q9t715
- Mulder FI, van Es N, Kraaijpoel N, Di Nisio M, Carrier M, Duggal A et al. Edoxaban for treatment of venous thromboembolism in patient groups with different types of cancer: Results from the Hokusai VTE Cancer study (vol 185, pg 13, 2020). Thrombosis Research. 2020;191:156-9. doi:10.1016/j.thromres.2019.11.007
- Oride T, Sawada K, Shimizu A, Kinose Y, Takiuchi T, Kodama M et al. Clinical trial assessing the safety of edoxaban with concomitant chemotherapy in patients with gynecological cancer-associated thrombosis (EGCAT study). Thrombosis Journal. 2023;21(1):57. doi:10.1186/s12959-023-00500-8
- Di Nisio M, van Es N, Carrier M, Wang TF, Garcia D, Segers A et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: A post‐hoc analysis of the Hokusai‐VTE Cancer study. Journal of Thrombosis and Haemostasis. 2019;17(11):1866-74. doi:10.1111/jth.14561
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al. Oral apixaban for the treatment of acute venous thromboembolism. New England Journal of Medicine. 2013;369(9):799-808. doi:10.1056/NEJMoa1302507
- Einstein Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. New England Journal of Medicine. 2010;363(26):2499-510. doi:10.1056/NEJMoa1007903
- Raskob GE, Van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. New England Journal of Medicine. 2018;378(7):615-24. doi:10.1056/NEJMoa1711948
- Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). Journal of Clinical Oncology. 2018 Jul 10;36(20):2017-23. doi:10.1200/JCO.2018.78.8034
- Abdullah HO, Abdalla BA, Kakamad FH, Ahmed JO, Baba HO, Hassan MN, et al. Predatory Publishing Lists: A Review on the Ongoing Battle Against Fraudulent Actions. Barw Medical Journal. 2024;2(2):26-30. doi:10.58742/bmj.v2i2.91
- Chatani R, Yamashita Y, Morimoto T, Muraoka N, Umetsu M, Nishimoto Y et al. Edoxaban for 12 Versus 3 Months in Cancer-associated Isolated Distal Deep Vein Thrombosis According to Different Doses: Insights from the ONCO DVT study. European Heart Journal-Cardiovascular Pharmacotherapy. 2024:pvae028. doi:10.1093/ehjcvp/pvae028
- Chung JW, Hwang J, Kim HJ, Seo WK, Ahn MJ, Saver JL, Bang OY. Edoxaban for the treatment of hypercoagulability and cerebral thromboembolism associated with cancer: a randomized clinical trial of biomarker targets. International Journal of Stroke. 2024: 19(6) 645–653. doi:10.1177/17474930241239266
- Nakamura M, Ishiguro A, Dazai M, Kawamoto Y, Yuki S, Sogabe S, et al. Feasibility of edoxaban for asymptomatic cancer-associated thrombosis in Japanese patients with gastrointestinal cancer: ExCAVE study. BMC cancer. 2022;22(1):13-22. doi:10.1186/s12885-022-10403-y
- Raskob GE, van Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z et al. Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. The Lancet Haematology. 2016;3(8):e379-87. doi:10.1016/S2352-3026(16)30057-6
- Yamashita Y, Morimoto T, Muraoka N, Oyakawa T, Umetsu M, Akamatsu D et al. Edoxaban for 12 months versus 3 months in patients with cancer with isolated distal deep vein thrombosis (ONCO DVT study): an open-label, multicenter, randomized clinical trial. Circulation. 2023;148(21):1665-76. doi:10.1161/CIRCULATIONAHA.123.066360
- Hara N, Miyamoto T, Iwai T, Yamaguchi J, Hijikata S, Watanabe K et al. Assessment of the safety and efficacy of edoxaban for treating venous thromboembolism secondary to active malignancy. Annals of Vascular Diseases. 2017;10(4):407-10. doi:10.3400/avd.oa.17-00054
- Ikeda S, Koga S, Yamagata Y, Eguchi M, Sato D, Muroya T et al. Comparison of the effects of edoxaban, an oral direct factor Xa inhibitor, on venous thromboembolism between patients with and without cancer. Journal of cardiology. 2018;72(2):120-7. doi:10.1016/j.jjcc.2018.03.006
- Cohen AL, Lim CS, Davies AH. Is there a role yet for new direct oral anticoagulants in cancer patients?. Phlebology. 2016(3):157-9. doi:10.1177/0268355515604255
- Rak J, Yu JL, Luyendyk J, Mackman N. Oncogenes, trousseau syndrome, and cancer-related changes in the coagulome of mice and humans. Cancer research. 2006;66(22):10643-6. doi:10.1158/0008-5472.CAN-06-2350
- Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clinical and applied thrombosis/hemostasis. 2006;12(4):389-96. doi:10.1177/1076029606293692
- Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. The American journal of medicine. 2006;119(12):1062-72. doi:10.1016/j.amjmed.2006.02.022
- Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. New England journal of medicine. 2003;349(2):146-53. doi:10.1056/NEJMoa025313
- Lee AY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. Jama. 2015;314(7):677-86. doi:10.1001/jama.2015.9243
- Prins MH, Lensing AW, Brighton TA, Lyons RM, Rehm J, Trajanovic M et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. The Lancet Haematology. 2014 Oct 1;1(1):e37-46. doi:10.1016/S2352-3026(14)70018-3

This work is licensed under a Creative Commons Attribution 4.0 International License.

