Breast Carcinoma within Fibroadenoma: A Systematic Review
Abstract
Introduction
Fibroadenoma is the most common benign breast lesion; however, it carries a potential risk of malignant transformation. This systematic review provides an overview of the presentation, management, and outcome of carcinomas arising within fibroadenomas.
Methods
A systematic search on Google Scholar was conducted for English-language studies on breast carcinoma within fibroadenomas. Studies on fibroadenomas with no malignant components, review articles, pre-prints, incomplete data, and those published in suspicious journals were excluded.
Results
On ultrasonography, 28 masses (36.8%) appeared benign, and 20 (26.3%) were suspicious, with ultrasonographic data unavailable for the remaining tumors (36.8%). Mammography data were available for 50 tumors, revealing 27 benign lesions (54%) and 23 suspicious lesions (46%). Among the 17 lesions with available magnetic resonance imaging data, five were benign lesions (29.4%), and 12 were suspicious (70.6%). Cytology evaluation among 46 tumors revealed that 20 (43.5%) were benign, 24 (52.2%) were malignant, and two (4.3%) were suspicious. The most commonly performed surgery was wide local excision (50.7%), followed by mastectomy (32.9%). On histopathology, 11 tumors exhibited more than one pathology. Ductal carcinoma in situ was the most frequent finding (40.8%), followed by invasive ductal carcinoma (28.9%) and lobular carcinoma in situ (28.4%). Recurrence was observed in one case (1.4%), and metastasis occurred in two cases (2.8%).
Conclusion
Although rare, carcinomas arising within fibroadenomas may present considerable challenges in preoperative diagnosis, whether through imaging or cytology. Therefore, clinicians may find it necessary to approach fibroadenomas with increased caution.
Introduction
Fibroadenoma is the most common benign breast lesion comprising epithelial and stromal components [1,2]. The tumor generally manifests as a hyperplastic breast lobule, presenting as a solitary mass during a woman’s early reproductive years, with the peak incidence occurring in the second and third decades of life [3,4]. Estrogen, progesterone, pregnancy, and lactation are believed to stimulate tumor growth, although it tends to shrink during menopause as estrogen levels decline [3]. Incidence rates range from 7% to 13% in the general population, with up to 20% of cases presenting with bilateral or multiple masses [3]. Clinically, fibroadenoma presents as a palpable, mobile, solid mass with a rubbery consistency and smooth, well-defined borders [5]. It is radiologically and histologically classified into simple and complex types [2]. The tumor may exceed 3 mm in size, be associated with sclerosing adenosis or epithelial calcifications, and potentially give rise to carcinomas that can invade the surrounding breast tissue. Although cases of fibroadenomas containing malignancies are rare, malignancy tends to occur more frequently in patients 10 to 20 years older than the typical age for simple fibroadenomas [2,6]. Carcinomas within fibroadenomas are most commonly carcinoma in situ (CIS) [7,8]. Invasive carcinomas, though less common, can occur, with invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) being the primary forms [6]. Carcinomas in situ signal an increased risk of developing invasive cancer if left untreated, and neoplasms arising within fibroadenomas behave similarly to those occurring independently [9]. This systematic review provides an overview of the presentation, management, and outcome of carcinomas arising within fibroadenomas.
Methods
Study design
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and search strategy
A systematic search on Google Scholar was undertaken to identify relevant English-language studies on breast carcinoma within fibroadenoma. The search strategy employed a combination of keywords, including "fibroadenoma" with terms such as (carcinoma, cancer, malignancy, malignant, carcinoma in situ, lobular carcinoma in situ (LCIS), and ductal carcinoma in situ (DCIS).
Eligibility criteria
The inclusion criteria were limited to studies specifically addressing breast carcinoma within fibroadenoma. Studies on fibroadenomas with no malignant components, review articles, pre-prints, incomplete data, and those published in suspicious journals were excluded [10].
Study selection and data extraction
Two authors independently reviewed the titles and abstracts of the identified publications. Following this, the same two authors assessed the full texts of the remaining studies based on predefined inclusion and exclusion criteria. The extracted data included the first author’s name, the country of publication, study design, patient demographics, clinical presentation, physical examination findings, imaging and cytology findings, treatment strategies, and disease prognosis.
Data analysis
Microsoft Excel (2019) was employed to collect and organize the extracted data, while data analysis (descriptive statistics) was performed using the Statistical Package for Social Sciences (SPSS), version 27.0. The results are presented as frequencies, percentages, ranges, mean with standard deviation, and medians with quartile ranges.
Results
Study selection and characteristics
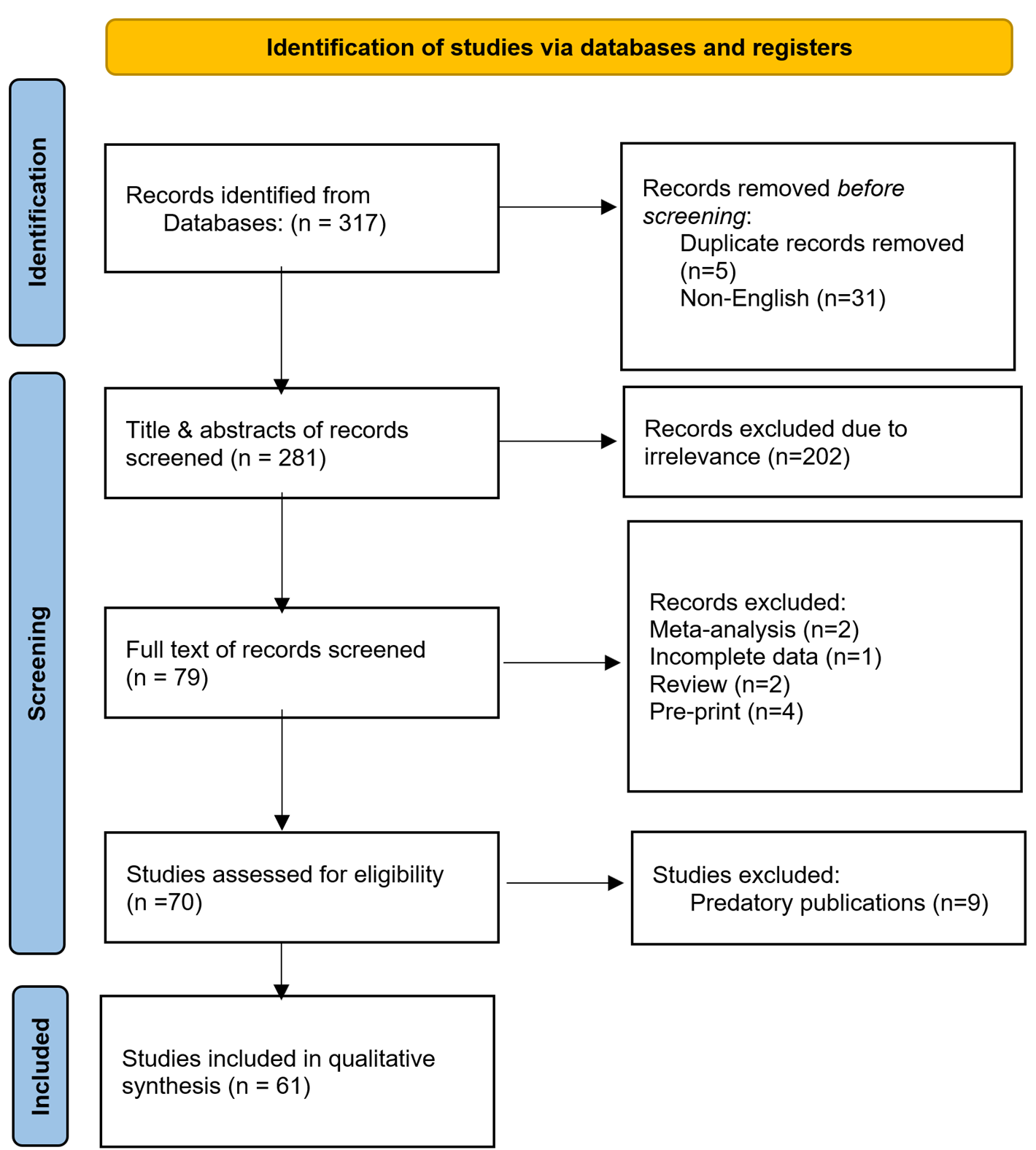
A total of 317 studies were identified from the search. Thirty-six studies were excluded due to duplication (n=5) and non-English language publications (n=31). This left 281 studies for title and abstract screening. At this stage, 202 studies were excluded due to irrelevancy. As a result, 79 studies advanced to the full-text screening stage. At this point, nine studies were excluded for being meta-analyses (n=2), reviews (n=2), publications with incomplete data (n=1), and pre-prints (n=4). Nine of the remaining 70 studies were excluded for failing to meet eligibility criteria as they were published in suspicious journals [10]. Ultimately, 61 studies [1-9,11-62], encompassing 72 cases, were included (Figure 1). Most of the studies were case reports (n=58), accompanied by three case series. Most were affiliated with Japan (19.7%) and the USA (14.7%) (Table 1). The raw data of the study has been presented in Tables 1-6.
| Author /Year [reference] | Study design | No. of included case(s) | Country |
|
Ni et al./2023 [14] |
Case report |
1 |
China |
|
Brunetti et al./2023 [4] |
Case report |
1 |
Italy |
|
Wang et al./2022 [5] |
Case report |
1 |
Singapore |
|
Pang et al./2022 [2] |
Case report |
1 |
Malaysia |
|
Hammood et al./2022 [3] |
Case report |
1 |
Iraq |
|
Tagliati et al./2021 [1] |
Case report |
1 |
Italy |
|
Shojaku et al./2021 [6] |
Case report |
1 |
Japan |
|
Fujimoto et al./2021 [11] |
Case report |
1 |
Japan |
|
Feijó et al./2021[8] |
Case report |
1 |
Brazil |
|
Shiino et al./2020 [12] |
Case report |
1 |
Japan |
|
Moreno et al./2020 [17] |
Case report |
1 |
Brazil |
|
Gonthong et al./2020 [13] |
Case report |
1 |
Thailand |
|
El-Essawy et al./2020 [18] |
Case report |
1 |
KSA |
|
Brock et al./2020 [9] |
Case report |
1 |
USA |
|
Marumoto et al./2019 [16] |
Case report |
1 |
USA |
|
Zeeshan et al./2018 [19] |
Case report |
1 |
Pakistan |
|
Tiwari et al./2018 [15] |
Case report |
1 |
India |
|
Frisch et al./2018 [7] |
Case report |
1 |
South Africa |
|
Lim et al./2017 [20] |
Case report |
1 |
Korea |
|
You et al./2016 [21] |
Case report |
1 |
Korea |
|
Zheng et al./2015 [22] |
Case report |
1 |
China |
|
Hua et al./2015 [23] |
Case report |
1 |
China |
|
Wu et al./2014 [24] |
Case series |
6 |
Taiwan |
|
Mele et al./2014 [25] |
Case report |
1 |
Denmark |
|
Limite et al./2014 [26] |
Case report |
1 |
Italy |
|
Kwon et al./2014 [27] |
Case report |
1 |
Korea |
|
Kılıç et al./2014 [28] |
Case report |
1 |
Turkey |
|
Dandin et al./2014 [29] |
Case report |
1 |
Turkey |
|
Buteau et al./2014 [30] |
Case report |
1 |
USA |
|
Hayes et al./2013 [31] |
Case report |
1 |
Ireland |
|
Jahan et al./2012 [32] |
Case report |
1 |
Bangladesh |
|
Butler et al./2012 [33] |
Case report |
1 |
USA |
|
Ooe et al./2011 [34] |
Case report |
1 |
Japan |
|
Lin et al./2011 [35] |
Case report |
1 |
Taiwan |
|
Kato et al./2011 [36] |
Case report |
1 |
Japan |
|
Abu-Rahmeh et al./ 2012 [37] |
Case report |
1 |
Israel |
|
Rao et al./ 2010 [38] |
Case report |
1 |
India |
|
Petersson et al./2010 [39] |
Case report |
1 |
Singapore |
|
Tajima et al./2009 [40] |
Case report |
1 |
Japan |
|
Gashi-Luci et al./2009 [41] |
Case report |
1 |
Kosova |
|
Borecky et al./2008 [42] |
Case series |
3 |
Australia |
|
Tiu et al./2006 [43] |
Case report |
1 |
Taiwan |
|
Shin et al./2006 [44] |
Case report |
1 |
Korea |
|
Blanco et al./2005 [45] |
Case report |
1 |
USA |
|
Abite et al./2005 [46] |
Case report |
1 |
Nigeria |
|
Stafyla et al./2004 [47] |
Case report |
1 |
Greece |
|
Abe et al./ 2004 [48] |
Case report |
1 |
Japan |
|
Adelekan et al./2003 [49] |
Case report |
1 |
UK |
|
Yano et al./2001 [50] |
Case report |
1 |
Japan |
|
Gebrim et al./2000 [51] |
Case report |
1 |
Brazil |
|
Psarianos et al./1998 [52] |
Case report |
1 |
Australia |
|
Shah et al./ 1998 [53] |
Case report |
1 |
USA |
|
Kurosum et al./1994 [54] |
Case report |
1 |
Japan |
|
Morimoto et al./1993 [55] |
Case report |
1 |
Japan |
|
Gupta et al./1992 [56] |
Case report |
1 |
New Zealand |
|
Gupta et al./1991 [57] |
Case report |
1 |
New Zealand |
|
Fukud et al./1989 [58] |
Case report |
1 |
Japan |
|
Yoshida et al./1985 [59] |
Case report |
1 |
Japan |
|
Fond et al./1979 [60] |
Case report |
1 |
USA |
|
Konakry et a./1975 [61] |
Case series |
5 |
USA |
|
Durso et al./1972 [62] |
Case report |
1 |
USA |
|
First Author /Year |
Age (years) |
Gender |
Presentation |
Laterality |
Duration (months) |
PMH |
FHx of breast cancer |
|
Ni et al./2023 [14] |
60 |
F |
Mass |
UL |
12 |
NN |
Neg. |
|
Brunetti et al./2023 [4] |
35 |
F |
Lump |
UL |
NA |
NN |
FDR |
|
Wang et al./2022 [5] |
26 |
F |
Lump |
UL |
72 |
NN |
NA |
|
Pang et al./2022 [2] |
43 |
F |
Nipple discharge |
UL |
NA |
BM |
Neg. |
|
Hammood et al./2022 [3] |
49 |
F |
Lump |
UL |
60 |
BM |
Neg. |
|
Tagliati et al./2021 [1] |
49 |
F |
Lump |
UL |
NA |
NA |
Neg. |
|
Shojaku et al./2021 [6] |
61 |
F |
Mass |
UL |
60 |
NN |
Neg. |
|
Fujimoto et al./2021 [11] |
31 |
F |
Mass |
UL |
12 |
NN |
Neg. |
|
Feijó et al./2021[8] |
31 |
F |
Lump |
UL |
48 |
NA |
Neg. |
|
Shiino et al./2020 [12] |
53 |
F |
Lump |
UL |
156 |
NA |
NA |
|
Moreno et al./2020 [17] |
58 |
F |
Lump |
UL |
NA |
NA |
NA |
|
Gonthong et al./2020 [13] |
38 |
F |
Mass |
UL |
NA |
IDC |
NA |
|
El-Essawy et al./2020 [18] |
25 |
F |
Mass |
UL |
1 |
MBBM |
Neg. |
|
Brock et al./2020 [9] |
27 |
F |
Lump |
UL |
4 |
FBD |
NA |
|
Marumoto et al./2019 [16] |
70 |
F |
Mass |
UL |
NA |
NA |
Neg. |
|
Zeeshan et al./2018 [19] |
34 |
F |
Lump |
UL |
12 |
NN |
NA |
|
Tiwari et al./2018 [15] |
28 |
F |
Lump |
BL |
96 |
NN |
Neg. |
|
Frisch et al./2018 [7] |
18 |
F |
Lump |
UL |
48 |
NN |
Neg. |
|
Lim et al./2017 [20] |
20 |
F |
Nodule |
UL |
NA |
NN |
Neg. |
|
You et al./2016 [21] |
38 |
F |
Incidental |
UL |
NA |
NA |
Neg. |
|
Zheng et al./2015 [22] |
48 |
F |
Lump |
BL |
NA |
NA |
NA |
|
Hua et al./2015 [23] |
44 |
F |
Lump |
BL |
12 |
NA |
NA |
|
Wu et al./2014 [24]
|
39 |
F |
NA |
NA |
24 |
NA |
NA |
|
31 |
F |
NA |
NA |
84 |
NA |
NA |
|
|
30 |
F |
NA |
NA |
NA |
NA |
NA |
|
|
63 |
F |
NA |
NA |
0.5 |
NA |
NA |
|
|
48 |
F |
NA |
NA |
3 |
NA |
NA |
|
|
40 |
F |
NA |
NA |
0 |
NA |
NA |
|
|
Mele et al./2014 [25] |
63 |
F |
NA |
UL |
NA |
NA |
Pos. |
|
Limite et al./2014 [26] |
26 |
F |
Lump |
UL |
NA |
NA |
Neg. |
|
Kwon et al./2014 [27] |
20 |
F |
Lump |
BL |
1 |
NN |
Neg. |
|
Kılıç et al./2014 [28] |
30 |
F |
Mass |
UL |
NA |
NA |
Neg. |
|
Dandin et al./2014 [29] |
35 |
F |
Mass |
UL |
1.5 |
NN |
Neg. |
|
Buteau et al./2014 [30] |
59 |
F |
Mass |
UL |
36 |
NN |
Neg. |
|
Hayes et al./2013 [31] |
51 |
F |
Incidental |
NA |
NA |
NA |
NA |
|
Jahan et al./2012 [32] |
55 |
F |
Lump |
BL |
240 |
NA |
NA |
|
Butler et al./2012 [33] |
46 |
F |
Mass |
NA |
60 |
NA |
NA |
|
Ooe et al./2011 [34] |
46 |
F |
Lump |
UL |
60 |
NN |
Neg. |
|
Lin et al./2011 [35] |
34 |
F |
Lump |
UL |
NA |
NN |
Neg. |
|
Kato et al./2011 [36] |
42 |
F |
Mass |
UL |
NA |
NA |
NA |
|
Abu-Rahmeh et al./ 2012 [37] |
69 |
F |
Mass |
UL |
168 |
NA |
FDR |
|
Rao et al./ 2010 [38] |
30 |
F |
Lump |
UL |
1 |
NN |
Neg. |
|
Petersson et al./2010 [39] |
49 |
F |
Incidental |
UL |
48 |
NA |
NA |
|
Tajima et al./2009 [40] |
60 |
F |
Mass |
UL |
3 |
NA |
NA |
|
Gashi-Luci et al./2009 [41] |
39 |
F |
Lump |
UL |
2 |
NA |
Neg. |
|
Borecky et al./2008 [42] |
64 |
F |
Mass |
UL |
NA |
NA |
NA |
|
80 |
F |
Lump |
UL |
600 |
NA |
NA |
|
|
53 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
Tiu et al./2006 [43] |
45 |
F |
Lump |
UL |
60 |
NN |
NA |
|
Shin et al./2006 [44] |
51 |
F |
Mass |
UL |
12 |
NN |
Neg. |
|
Blanco et al./2005 [45] |
63 |
F |
Mass |
UL |
60 |
NN |
Neg. |
|
Abite et al./2005 [46] |
23 |
F |
Lump |
UL |
12 |
NA |
Neg. |
|
Stafyla et al./2004 [47] |
27 |
F |
Mass |
UL |
NA |
NA |
NA |
|
Abe et al./ 2004 [48] |
42 |
F |
Lump |
UL |
3 |
NN |
Neg. |
|
Adelekan et al./2003 [49] |
61 |
F |
Lump |
BL |
120, 0.75 |
NA |
NA |
|
Yano et al./2001 [50] |
54 |
F |
Mass |
UL |
36 |
NA |
Neg. |
|
Gebrim et al./2000 [51] |
58 |
F |
Nodule |
UL |
NA |
NA |
NA |
|
Psarianos et al./1998 [52] |
34 |
F |
Mass |
UL |
NA |
NA |
NA |
|
Shah et al./ 1998 [53] |
45 |
F |
Mass |
UL |
0.25 |
NA |
Neg. |
|
Kurosum et al./1994 [54] |
42 |
F |
Lump |
UL |
21 |
NA |
NA |
|
Morimoto et al./1993 [55] |
49 |
F |
Lump |
UL |
2 |
NA |
NA |
|
Gupta et al./1992 [56] |
59 |
F |
Mass |
UL |
0.5 |
NN |
Neg. |
|
Gupta et al./1991 [57] |
49 |
F |
Mass |
UL |
7 |
NA |
Neg. |
|
Fukud et al./1989 [58] |
45 |
F |
Lump |
UL |
NA |
BM |
NA |
|
Yoshida et al./1985 [59] |
58 |
F |
Lump |
UL |
1 |
HTN |
Neg. |
|
Fond et al./1979 [60] |
27 |
F |
Lump |
UL |
NA |
CAH |
SDR |
|
Konakry et a./1975 [61] |
59 |
F |
NA |
UL |
NA |
NA |
NA |
|
39 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
44 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
46 |
F |
NA |
UL |
NA |
DCIS |
NA |
|
|
48 |
F |
NA |
UL |
NA |
NA |
NA |
|
|
Durso et al./1972 [62] |
42 |
F |
Lump |
UL |
NA |
NA |
NA |
| F: female, PMH: Past Medical History, FHx: Family History, UL: Unilateral, BL: bilateral, NA: Non-available, BM: Breast Mass, NN: Nothing Noteworthy, IDC: Invasive Ductal Carcinoma, MBBM: Multiple Bilateral Breast Mass, FBD: Fibrocystic Breast Disease, HTN: Hypertension, CAH: Congenital Adrenal Hyperplasia, DCIS: Ductal Carcinoma In Situ, FDR: First-Degree Relative, SDR: Second-Degree Relative, Neg.: Negative, Pos.: Positive. | |||||||
| First Author. /Year | Physical examination |
Ax LAD |
Size |
Location |
Shape |
Margin |
Vascularity |
Calcification |
||
|
Surface |
Consistency |
Mobility |
||||||||
|
Ni et al./2023 [14] |
NA |
NA |
NM |
Neg. |
7.7 mm |
RUA |
Round |
Smooth |
NA |
Pos. |
|
Brunetti et al./2023 [4] |
NA |
NA |
M |
Neg. |
15 mm |
LLA |
Oval |
Well defined |
NA |
NA |
|
Wang et al./2022 [5] |
NA |
NA |
NA |
NA |
24 mm |
LT |
NA |
Irregular |
NA |
NA |
|
Pang et al./2022 [2] |
NA |
NA |
NA |
NA |
16.7 mm |
ROA |
Oval |
Lobulated |
Moderate |
Neg. |
|
Hammood et al./2022 [3] |
Smooth |
Firm |
NM |
NA |
9.5mm |
RT |
Oval |
Well defined |
NA |
NA |
|
Tagliati et al./2021 [1] |
NA |
NA |
NA |
NA |
35 mm |
RT |
Oval |
Well defined |
NA |
NA |
|
Shojaku et al./2021 [6] |
NA |
Hard |
NA |
NA |
11.9 mm |
LT |
Oval |
Well defined |
NA |
Neg. |
|
Fujimoto et al./2021 [11] |
NA |
NA |
NA |
Neg. |
22 mm |
LT |
NA |
Well defined |
NA |
Pos. |
|
Feijó et al./2021[8] |
NA |
NA |
NA |
Neg. |
30 mm |
LUOQ |
NA |
Well defined |
Neg. |
Neg. |
|
Shiino et al./2020 [12] |
NA |
Hard |
NA |
Pos. |
36 mm |
RLIQ |
NA |
Ill defined |
NA |
Pos. |
|
Moreno et al./2020 [17] |
NA |
NA |
NA |
Pos. |
9.8 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
Gonthong et al./2020 [13] |
NA |
NA |
NA |
NA |
20 mm |
RT |
Oval |
Microlobulated |
NA |
Pos. |
|
El-Essawy et al./2020 [18] |
NA |
NA |
NA |
NA |
28.7 mm |
LIA |
NA |
Irregular |
Increased |
Pos. |
|
Brock et al./2020 [9] |
NA |
Firm |
M |
NA |
19.8 mm |
LOA |
NA |
NA |
NA |
Neg. |
|
Marumoto et al./2019 [16] |
NA |
NA |
M |
Neg. |
20.4 mm |
RUOQ |
NA |
Irregular |
NA |
Neg. |
|
Zeeshan et al./2018 [19] |
NA |
NA |
M |
NA |
47.9 mm |
RRA |
NA |
Lobulated |
NA |
NA |
|
Tiwari et al./2018 [15] |
Smooth |
Firm |
M |
NA |
NA |
BL |
NA |
Well defined |
NA |
NA |
|
Frisch et al./2018 [7] |
NA |
NA |
M |
Neg. |
39.3 mm |
RLIQ |
NA |
Well defined |
Neg. |
Neg. |
|
Lim et al./2017 [20] |
NA |
NA |
NA |
NA |
64.8 mm |
RUA |
NA |
NA |
NA |
NA |
|
You et al./2016 [21] |
NA |
NA |
NA |
Neg. |
6.9 mm |
RUIQ |
Oval |
Well defined |
NA |
Pos. |
|
Zheng et al./2015 [22] |
NA, Smooth |
NA, NA |
NA, M |
Neg., Neg. |
24.5 mm, NA |
LUA, RUIQ |
NA, NA |
Ill defined, well defined |
NA, NA |
NA, Pos. |
|
Hua et al./2015 [23] |
NA |
NA |
NA |
NA |
22.4 mm |
LT |
NA |
Well defined |
Moderate |
Pos. |
|
Wu et al./2014 [24] |
NA |
NA |
NA |
NA |
27 mm |
NA |
NA |
NA |
NA |
NA |
|
NA |
NA |
NA |
NA |
34.5 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
14.5 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
12 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
9 mm |
NA |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
6 mm |
NA |
NA |
NA |
NA |
NA |
|
|
Mele et al./2014 [25] |
NA |
NA |
NA |
Pos. |
50 mm |
LLOQ |
NA |
Well defined |
NA |
Pos. |
|
Limite et al./2014 [26] |
Smooth |
Hard |
M |
Neg. |
1.8 mm |
RLA |
NA |
Ill defined |
NA |
NA |
|
Kwon et al./2014 [27] |
NA, NA |
Firm, Firm |
M, M |
Neg., Neg. |
16.9 mm, 21.9 mm |
RT, LT |
NA, Oval |
Lobulated, Irregular |
NA, NA |
Pos., Pos. |
|
Kılıç et al./2014 [28] |
NA |
Firm |
NA |
Neg. |
19.9 mm |
LRA |
NA |
Well defined |
NA |
Pos. |
|
Dandin et al./2014 [29] |
NA |
NA |
M |
Neg. |
11.8 mm |
LUOQ |
Oval |
Irregular |
NA |
NA |
|
Buteau et al./2014 [30] |
NA |
NA |
NA |
Pos. |
17 mm |
LT |
Lobular |
NA |
NA |
NA |
|
Hayes et al./2013 [31] |
NA |
NA |
NA |
NA |
35 mm |
NA |
Multilobulated |
Circumscribed |
NA |
Pos. |
|
Jahan et al./2012 [32] |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
39.2 mm, 36.3 mm |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
|
Butler et al./2012 [33] |
NA |
NA |
NA |
NA |
7.3 mm |
NA |
Oval |
Well defined |
NA |
NA |
|
Ooe et al./2011 [34] |
Smooth |
Firm |
M |
Neg. |
25 mm |
RUOQ |
Oval |
Well defined |
Increased |
Pos. |
|
Lin et al./2011 [35] |
NA |
NA |
M |
Neg. |
NA |
RUA |
Oval |
Well defined |
NA |
Pos. |
|
Kato et al./2011 [36] |
NA |
Hard |
NA |
NA |
15 mm |
RT |
Irregular |
NA |
NA |
Pos. |
|
Abu-Rahmeh et al./ 2012 [37] |
NA |
NA |
NA |
NA |
50 mm |
LT |
NA |
Well defined |
NA |
NA |
|
Rao et al./ 2010 [38] |
NA |
Firm |
M |
NA |
28.3 mm |
RUA |
Oval |
Smooth |
NA |
Pos. |
|
Petersson et al./2010 [39] |
NA |
NA |
NA |
NA |
30 mm |
NA |
NA |
Well defined |
NA |
NA |
|
Tajima et al./2009 [40] |
NA |
NA |
M |
NA |
16.6 mm |
RUIQ |
Lobular |
Irregular |
NA |
Pos. |
|
Gashi-Luci et al./2009 [41] |
NA |
NA |
NA |
Neg. |
20 mm |
RUOQ |
NA |
NA |
NA |
NA |
|
Borecky et al./2008 [42] |
NA |
NA |
NA |
NA |
12 mm |
LT |
NA |
Irregular |
NA |
Pos. |
|
NA |
NA |
NA |
NA |
40 mm |
LUIQ |
NA |
Ill defined |
NA |
Pos. |
|
|
NA |
NA |
NA |
NA |
17 mm |
NA |
Oval |
Well defined |
NA |
Pos. |
|
|
Tiu et al./2006 [43] |
NA |
NA |
M |
Neg. |
13 mm |
LUOQ |
NA |
Well defined |
Increased |
NA |
|
Shin et al./2006 [44] |
NA |
NA |
M |
Neg. |
12.3 mm |
RUIQ |
Oval |
Well defined |
Pos. |
Pos. |
|
Blanco et al./2005 [45] |
NA |
NA |
NA |
NA |
17.5 mm |
RT |
Round |
Well defined |
NA |
Pos. |
|
Abite et al./2005 [46] |
NA |
Firm |
M |
Neg. |
34.2 mm |
RUOQ |
NA |
Well defined |
NA |
NA |
|
Stafyla et al./2004 [47] |
NA |
NA |
M |
Neg. |
34 mm |
RUOQ |
NA |
Well defined |
NA |
NA |
|
Abe et al./ 2004 [48] |
NA |
Firm |
NA |
Neg. |
47.4 mm |
LUOQ |
Irregular |
Well defined |
NA |
Neg. |
|
Adelekan et al./2003 [49] |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
35 mm, 60 mm |
NA, LUIQ |
NA, NA |
NA, NA |
NA, NA |
NA, NA |
|
Yano et al./2001 [50] |
Smooth |
Hard |
M |
Neg. |
18.8 mm |
LUIQ |
NA |
Well defined |
Minimal |
Neg. |
|
Gebrim et al./2000 [51] |
NA |
NA |
M |
Neg. |
24.5 mm |
LT |
NA |
Well defined |
NA |
Neg. |
|
Psarianos et al./1998 [52] |
NA |
Firm |
M |
NA |
29.7 mm |
RUIQ |
NA |
Well defined |
NA |
NA |
|
Shah et al./ 1998 [53] |
NA |
Firm |
M |
Neg. |
22.4 mm |
RUIQ |
NA |
Well defined |
NA |
NA |
|
Kurosum et al./1994 [54] |
NA |
Rubbery |
NA |
Neg. |
22.9 mm |
RUOQ |
NA |
Well defined |
NA |
NA |
|
Morimoto et al./1993 [55] |
NA |
Rubbery |
M |
NA |
24.5 mm |
LUIQ |
NA |
Well defined |
NA |
NA |
|
Gupta et al./1992 [56] |
NA |
Firm |
NA |
NA |
19.4 mm |
LT |
NA |
NA |
NA |
NA |
|
Gupta et al./1991 [57] |
NA |
Rubbery |
NA |
NA |
NA |
LUOQ |
NA |
NA |
NA |
NA |
|
Fukud et al./1989 [58] |
Smooth |
NA |
NA |
NA |
39.2 mm |
ROA |
NA |
NA |
NA |
Neg. |
|
Yoshida et al./1985 [59] |
Smooth |
Firm |
PM |
Neg. |
34.1 mm |
LUOQ |
NA |
Well defined |
High |
Neg. |
|
Fond et al./1979 [60] |
NA |
NA |
M |
NA |
20 mm |
RSA |
NA |
NA |
NA |
NA |
|
Konakry et a./1975 [61] |
NA |
NA |
NA |
NA |
20 mm |
RUOQ |
NA |
NA |
NA |
Pos. |
|
NA |
NA |
NA |
NA |
50 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
20 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
8 mm |
RUOQ |
NA |
NA |
NA |
NA |
|
|
NA |
NA |
NA |
NA |
31.1 mm |
LUOQ |
NA |
NA |
NA |
NA |
|
|
Durso et al./1972 [62] |
Smooth |
NA |
NA |
NA |
15 mm |
RUIQ |
NA |
NA |
NA |
NA |
| N/A: Non-available, mm: Millimeters, Ax LAD: Axillary Lymphadenopathy, RUA: Right Upper Aspect, LLA: Left Lower Aspect, LT: Left, RT: Right, ROA: Right Outer Aspect, LUIQ: Left Upper Inner Quadrant, RLOQ: Right Lower Outer Quadrant, LUOQ: Left Upper Outer Quadrant, RLIQ: Right Lower Inner Quadrant, LIA: Left Inner Aspect, LOA: Left Outer Aspect, RUOQ: Right Upper Outer Quadrant, RRA: Right Retro-Areolar, BL: Bilateral, RLA: Right Lower Aspect, LRA: Left Retro-Areolar, LLOQ: Left Lower Outer Quadrant, LUA: Left Upper Aspect, RIA: Right Inner Aspect, RUIQ: Right Upper Inner Quadrant, RSA: Right Subareolar Area, Neg.: Negative, Pos.: Positive, NM: Non-Mobile, M: Mobile, PM: Partially mobile. | ||||||||||
| First Author. /Year |
Radiological findings |
Pre-operative diagnosis (CNB or FNAC) | ||
|
U/S |
MMG |
MRI |
||
|
Ni et al./2023 [14] |
Benign |
Benign |
Suspicious |
N/A |
|
Brunetti et al./2023 [4] |
Suspicious |
Benign |
N/A |
DCIS |
|
Wang et al./2022 [5] |
Benign |
N/A |
N/A |
Benign |
|
Pang et al./2022 [2] |
Benign |
Benign |
N/A |
Benign |
|
Hammood et al./2022 [3] |
Benign |
Benign |
Benign |
Benign |
|
Tagliati et al./2021 [1] |
Benign |
N/A |
Suspicious |
Benign |
|
Shojaku et al./2021 [6] |
Benign |
Benign |
Suspicious |
Malignant |
|
Fujimoto et al./2021 [11] |
Suspicious |
Suspicious |
Benign |
IDC |
|
Feijó et al./2021[8] |
Benign |
N/A |
N/A |
Suspicious |
|
Shiino et al./2020 [12] |
Suspicious |
Suspicious |
Suspicious |
IDC |
|
Moreno et al./2020 [17] |
N/A |
N/A |
N/A |
N/A |
|
Gonthong et al./2020 [13] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
El-Essawy et al./2020 [18] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
Brock et al./2020 [9] |
Benign |
Benign |
N/A |
Benign |
|
Marumoto et al./2019 [16] |
Suspicious |
Benign |
N/A |
Benign |
|
Zeeshan et al./2018 [19] |
Suspicious |
Suspicious |
N/A |
Benign |
|
Tiwari et al./2018 [15] |
Benign |
N/A |
N/A |
Benign |
|
Frisch et al./2018 [7] |
Benign |
N/A |
N/A |
N/A |
|
Lim et al./2017 [20] |
N/A |
N/A |
N/A |
N/A |
|
You et al./2016 [21] |
Suspicious |
Suspicious |
N/A |
Suspicious |
|
Zheng et al./2015 [22] |
Suspicious, Benign |
N/A, N/A |
N/A, N/A |
N/A, N/A |
|
Hua et al./2015 [23] |
Suspicious |
Suspicious |
N/A |
Benign |
|
Wu et al./2014 [24]
|
N/A |
N/A |
N/A |
N/A |
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
N/A |
N/A |
N/A |
N/A |
|
|
Mele et al./2014 [25] |
Benign |
Suspicious |
Suspicious |
IAC |
|
Limite et al./2014 [26] |
Benign |
N/A |
N/A |
N/A |
|
Kwon et al./2014 [27] |
Benign, Benign |
N/A, N/A |
N/A, N/A |
Benign, Benign |
|
Kılıç et al./2014 [28] |
Benign |
Suspicious |
Benign |
DCIS |
|
Dandin et al./2014 [29] |
Suspicious |
N/A |
N/A |
N/A |
|
Buteau et al./2014 [30] |
N/A |
Benign |
Benign |
Benign |
|
Hayes et al./2013 [31] |
N/A |
Suspicious |
N/A |
Benign |
|
Jahan et al./2012 [32] |
Benign, Benign |
N/A, N/A |
N/A, N/A |
N/A, N/A |
|
Butler et al./2012 [33] |
Benign |
Benign |
N/A |
ILC – LCIS |
|
Ooe et al./2011 [34] |
Suspicious |
Benign |
Suspicious |
DCIS |
|
Lin et al./2011 [35] |
Benign |
Suspicious |
N/A |
IDC - DCIS |
|
Kato et al./2011 [36] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
Abu-Rahmeh et al./ 2012 [37] |
Benign |
Benign |
N/A |
IDC |
|
Rao et al./ 2010 [38] |
Benign |
Benign |
N/A |
Malignant |
|
Petersson et al./2010 [39] |
N/A |
Benign |
N/A |
N/A |
|
Tajima et al./2009 [40] |
Suspicious |
Suspicious |
Suspicious |
Malignant |
|
Gashi-Luci et al./2009 [41] |
Suspicious |
Suspicious |
N/A |
Benign |
|
Borecky et al./2008 [42] |
Suspicious |
Suspicious |
N/A |
IDC - DCIS |
|
Suspicious |
Suspicious |
N/A |
IC |
|
|
Suspicious |
Suspicious |
N/A |
IDC - DCIS |
|
|
Tiu et al./2006 [43] |
Benign |
Benign |
N/A |
Malignant |
|
Shin et al./2006 [44] |
Suspicious |
Suspicious |
Suspicious |
DCIS |
|
Blanco et al./2005 [45] |
N/A |
Benign |
N/A |
N/A |
|
Abite et al./2005 [46] |
N/A |
N/A |
N/A |
N/A |
|
Stafyla et al./2004 [47] |
Benign |
N/A |
N/A |
N/A |
|
Abe et al./ 2004 [48] |
Suspicious |
Benign |
N/A |
Benign |
|
Adelekan et al./2003 [49] |
N/A, N/A |
Benign, Benign |
N/A, N/A |
Benign, Malignant |
|
Yano et al./2001 [50] |
Benign |
Suspicious |
Benign |
Malignant |
|
Gebrim et al./2000 [51] |
N/A |
Suspicious |
N/A |
Benign |
|
Psarianos et al./1998 [52] |
Benign |
Benign |
N/A |
N/A |
|
Shah et al./ 1998 [53] |
N/A |
Benign |
N/A |
Benign |
|
Kurosum et al./1994 [54] |
Benign |
N/A |
N/A |
N/A |
|
Morimoto et al./1993 [55] |
N/A |
N/A |
N/A |
Benign |
|
Gupta et al./1992 [56] |
N/A |
Suspicious |
N/A |
Malignant |
|
Gupta et al./1991 [57] |
N/A |
Benign |
N/A |
Malignant |
|
Fukud et al./1989 [58] |
Benign |
Benign |
N/A |
N/A |
|
Yoshida et al./1985 [59] |
N/A |
Suspicious |
Suspicious |
N/A |
|
Fond et al./1979 [60] |
N/A |
N/A |
N/A |
Benign |
|
Konakry et a./1975 [61] |
N/A |
Suspicious |
N/A |
N/A |
|
N/A |
Benign |
N/A |
N/A |
|
|
N/A |
Benign |
N/A |
N/A |
|
|
N/A |
Benign |
N/A |
N/A |
|
|
N/A |
Benign |
N/A |
N/A |
|
|
Durso et al./1972 [62] |
N/A |
Benign |
N/A |
N/A |
| N/A: non-available, U/S: Ultrasound, MMG: Mammogram, MRI: Magnetic Resonance Imaging, CNB: Core Needle Biopsy, FNAC: Fine Needle Aspiration Cytology, DCIS: Ductal Carcinoma In Situ, IDC: Invasive Ductal Carcinoma, CIS: Carcinoma In Situ, IAC: Invasive apocrine carcinoma, ILC: Invasive Lobular Carcinoma, LCIS: Lobular Carcinoma In Suspicious, IC: Invasive Carcinoma. | ||||
|
First Author /Year |
Management |
Hormonal therapy |
|||
|
Breast surgery |
Axillary surgery |
Chemotherapy |
Radiotherapy | ||
|
Ni et al./2023 [14] |
WLE |
SLNB |
No |
No |
NA |
|
Brunetti et al./2023 [4] |
WLE |
ALND |
Yes |
NA |
NA |
|
Wang et al./2022 [5] |
EB |
None |
NA |
Yes |
Yes |
|
Pang et al./2022 [2] |
WLE |
None |
No |
NA |
Yes |
|
Hammood et al./2022 [3] |
WLE |
None |
NA |
NA |
Yes |
|
Tagliati et al./2021 [1] |
WLE |
None |
NA |
No |
NA |
|
Shojaku et al./2021 [6] |
WLE |
SLNB |
NA |
Yes |
NA |
|
Fujimoto et al./2021 [11] |
WLE |
SLNB |
Yes |
Yes |
NA |
|
Feijó et al./2021[8] |
WLE |
None |
Yes |
Yes |
NA |
|
Shiino et al./2020 [12] |
MX |
ALND |
Yes |
Yes |
NA |
|
Moreno et al./2020 [17] |
MX |
None |
NA |
NA |
NA |
|
Gonthong et al./2020 [13] |
MX |
ALND |
No |
No |
Yes |
|
El-Essawy et al./2020 [18] |
WLE |
None |
NA |
NA |
NA |
|
Brock et al./2020 [9] |
WLE |
None |
NA |
NA |
NA |
|
Marumoto et al./2019 [16] |
EB |
None |
No |
Yes |
Yes |
|
Zeeshan et al./2018 [19] |
WLE |
None |
NA |
Yes |
Yes |
|
Tiwari et al./2018 [15] |
WLE |
None |
No |
No |
NA |
|
Frisch et al./2018 [7] |
WLE |
NA |
NA |
No |
Yes |
|
Lim et al./2017 [20] |
WLE |
None |
No |
No |
No |
|
You et al./2016 [21] |
WLE |
None |
NA |
NA |
Yes |
|
Zheng et al./2015 [22] |
MX |
ALND |
Yes |
NA |
Yes |
|
Hua et al./2015 [23] |
MX |
None |
NA |
NA |
Yes |
|
Wu et al./2014 [24]
|
WLE |
SLNB |
No |
No |
Yes |
|
MX |
ALND |
Yes |
No |
Yes |
|
|
WLE |
NA |
No |
No |
Yes |
|
|
WLE |
SLNB |
No |
Yes |
Yes |
|
|
WLE |
SLNB |
No |
No |
No |
|
|
MX |
SLNB |
No |
No |
Yes |
|
|
Mele et al./2014 [25] |
MRM |
ALND |
NA |
NA |
NA |
|
Limite et al./2014 [26] |
WLE |
SLNB |
No |
No |
NA |
|
Kwon et al./2014 [27] |
WLE |
None |
NA |
Yes |
NA |
|
Kılıç et al./2014 [28] |
WLE |
None |
NA |
NA |
NA |
|
Dandin et al./2014 [29] |
WLE |
ALND |
Yes |
NA |
NA |
|
Buteau et al./2014 [30] |
WLE |
ALND |
Yes |
Yes |
Yes |
|
Hayes et al./2013 [31] |
WLE |
SLNB |
NA |
NA |
NA |
|
Jahan et al./2012 [32] |
WLE |
None |
NA |
NA |
NA |
|
Butler et al./2012 [33] |
WLE |
None |
NA |
NA |
NA |
|
Ooe et al./2011 [34] |
WLE |
SLNB |
No |
Yes |
Yes |
|
Lin et al./2011 [35] |
MRM |
None |
NA |
NA |
NA |
|
Kato et al./2011 [36] |
WLE |
SLNB |
NA |
NA |
NA |
|
Abu-Rahmeh et al./ 2012 [37] |
NA |
NA |
NA |
NA |
NA |
|
Rao et al./ 2010 [38] |
MRM |
ALND |
NA |
NA |
NA |
|
Petersson et al./2010 [39] |
EB |
SLNB |
NA |
NA |
NA |
|
Tajima et al./2009 [40] |
WLE |
None |
NA |
NA |
NA |
|
Gashi-Luci et al./2009 [41] |
RM |
ALND |
NA |
NA |
NA |
|
Borecky et al./2008 [42] |
NA |
NA |
NA |
NA |
NA |
|
NA |
NA |
NA |
NA |
NA |
|
|
EB |
SLNB |
NA |
NA |
NA |
|
|
Tiu et al./2006 [43] |
MX |
None |
NA |
NA |
NA |
|
Shin et al./2006 [44] |
MX |
SLNB |
NA |
NA |
NA |
|
Blanco et al./2005 [45] |
WLE |
SLNB |
NA |
NA |
NA |
|
Abite et al./2005 [46] |
EB |
None |
NA |
NA |
NA |
|
Stafyla et al./2004 [47] |
EB |
None |
No |
No |
NA |
|
Abe et al./ 2004 [48] |
MX |
ALND |
Yes |
NA |
Yes |
|
Adelekan et al./2003 [49] |
EB, MRM |
None, ALND |
Yes |
Yes |
Yes |
|
Yano et al./2001 [50] |
WLE |
ALND |
NA |
Yes |
NA |
|
Gebrim et al./2000 [51] |
MX |
ALND |
NA |
NA |
NA |
|
Psarianos et al./1998 [52] |
EB |
None |
NA |
NA |
NA |
|
Shah et al./ 1998 [53] |
WLE |
NA |
NA |
NA |
NA |
|
Kurosum et al./1994 [54] |
WLE |
None |
NA |
Yes |
NA |
|
Morimoto et al./1993 [55] |
WLE |
None |
Yes |
NA |
NA |
|
Gupta et al./1992 [56] |
WLE |
None |
NA |
Yes |
Yes |
|
Gupta et al./1991 [57] |
WLE |
ALND |
NA |
Yes |
NA |
|
Fukud et al./1989 [58] |
MRM |
NA |
NA |
NA |
NA |
|
Yoshida et al./1985 [59] |
RM |
ALND |
No |
No |
NA |
|
Fond et al./1979 [60] |
MRM |
ALND |
NA |
NA |
NA |
|
Konakry et a./1975 [61] |
RM |
NA |
NA |
NA |
NA |
|
MRM |
NA |
NA |
NA |
NA |
|
|
MRM |
NA |
NA |
NA |
NA |
|
|
MX |
NA |
NA |
NA |
NA |
|
|
MRM |
NA |
NA |
NA |
NA |
|
|
Durso et al./1972 [62] |
EB |
None |
NA |
NA |
NA |
| NA: non-available, WLE: Wide Local Excision, EB: Excisional Biopsy, RM: Radical Mastectomy, MRM: Modified Radical Mastectomy, MX: Mastectomy, ALND: Axillary Lymph Node Dissection, SLNB: Sentinel Lymph Node Biopsy. | |||||
|
First Author /Year |
Post-operative HPE |
Immunohistochemistry (ER-PR-HER2) |
Axillary status |
FU (months) |
Recurrence |
Metastasis |
|
Ni et al./2023 [14] |
DCIS |
ER - PR |
Neg. |
NA |
NA |
No |
|
Brunetti et al./2023 [4] |
IDC |
TN |
Pos. |
NA |
NA |
Yes |
|
Wang et al./2022 [5] |
ILC - LCIS |
ER – PR |
NA |
NA |
NA |
NA |
|
Pang et al./2022 [2] |
LCIS |
NA |
NA |
4 |
No |
No |
|
Hammood et al./2022 [3] |
DCIS |
NA |
NA |
NA |
No |
No |
|
Tagliati et al./2021 [1] |
DCIS |
ER – PR |
NA |
60 |
No |
No |
|
Shojaku et al./2021 [6] |
DCIS |
ER |
NA |
24 |
No |
No |
|
Fujimoto et al./2021 [11] |
IDC |
HER2 |
Neg. |
6 |
No |
No |
|
Feijó et al./2021[8] |
DCIS |
ER – PR |
NA |
48 |
No |
No |
|
Shiino et al./2020 [12] |
IDC |
TN |
Neg. |
30 |
No |
No |
|
Moreno et al./2020 [17] |
LCIS |
NA |
NA |
120 |
No |
No |
|
Gonthong et al./2020 [13] |
DCIS |
TN |
Neg. |
12 |
No |
No |
|
El-Essawy et al./2020 [18] |
NA |
TN |
NA |
NA |
NA |
NA |
|
Brock et al./2020 [9] |
LCIS |
NA |
NA |
NA |
NA |
NA |
|
Marumoto et al./2019 [16] |
DCIS |
ER |
NA |
12 |
No |
No |
|
Zeeshan et al./2018 [19] |
DCIS |
ER – PR |
NA |
NA |
NA |
No |
|
Tiwari et al./2018 [15] |
DCIS |
NA |
NA |
12 |
No |
No |
|
Frisch et al./2018 [7] |
DCIS |
ER |
NA |
NA |
NA |
No |
|
Lim et al./2017 [20] |
CA |
TN |
NA |
21 |
No |
No |
|
You et al./2016 [21] |
DCIS |
ER – PR |
NA |
52 |
No |
No |
|
Zheng et al./2015 [22] |
ILC, IDC |
HER2, ER-PR-HER2 |
Neg., Neg. |
3 |
No |
No |
|
Hua et al./2015 [23] |
LCIS |
ER – PR |
NA |
60 |
No |
No |
|
Wu et al./2014 [24]
|
IDC |
ER – PR |
Neg. |
NA |
NA |
NA |
|
IDC |
ER – PR |
Pos. |
NA |
NA |
NA |
|
|
DCIS |
ER – PR |
NA |
NA |
NA |
NA |
|
|
DCIS |
ER – PR |
Neg. |
NA |
NA |
NA |
|
|
DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
|
IDC |
ER – PR |
Neg. |
NA |
NA |
NA |
|
|
Mele et al./2014 [25] |
IAC |
HER2 |
Pos. |
NA |
NA |
NA |
|
Limite et al./2014 [26] |
ACC (Ac) |
TN |
Neg. |
8 |
No |
No |
|
Kwon et al./2014 [27] |
DCIS, DCIS |
ER – PR, ER - PR |
NA, NA |
NA, NA |
NA |
NA |
|
Kılıç et al./2014 [28] |
DCIS |
NA |
NA |
NA |
NA |
NA |
|
Dandin et al./2014 [29] |
IDC - ILC - DCIS |
PR - HER2 |
Neg. |
6 |
No |
No |
|
Buteau et al./2014 [30] |
ILC |
NA |
Pos. |
NA |
No |
No |
|
Hayes et al./2013 [31] |
ILC |
ER |
Neg. |
NA |
NA |
NA |
|
Jahan et al./2012 [32] |
IDC, IDC |
NA, NA |
NA, NA |
NA, NA |
NA |
NA |
|
Butler et al./2012 [33] |
ILC - LCIS |
NA |
NA |
NA |
NA |
NA |
|
Ooe et al./2011 [34] |
DCIS |
ER – PR |
Neg. |
6 |
No |
No |
|
Lin et al./2011 [35] |
IDC - DCIS |
ER – PR |
NA |
24 |
No |
No |
|
Kato et al./2011 [36] |
DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
Abu-Rahmeh et al./ 2012 [37] |
IDC |
NA |
NA |
NA |
NA |
Yes |
|
Rao et al./ 2010 [38] |
IDC |
TN |
Pos. |
NA |
NA |
NA |
|
Petersson et al./2010 [39] |
IDC - DCIS |
ER – PR |
Neg. |
24 |
No |
No |
|
Tajima et al./2009 [40] |
ILC - LCIS |
ER |
NA |
NA |
NA |
NA |
|
Gashi-Luci et al./2009 [41] |
IDC - DCIS |
HER2 |
Neg. |
5 |
Yes |
NA |
|
Borecky et al./2008 [42] |
IDC - DCIS |
ER – PR |
Neg. |
NA |
NA |
NA |
|
IDC |
NA |
Neg. |
NA |
NA |
NA |
|
|
IDC - DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
|
Tiu et al./2006 [43] |
DCIS |
NA |
NA |
18 |
No |
No |
|
Shin et al./2006 [44] |
IDC - DCIS |
ER – PR |
Neg. |
16 |
No |
No |
|
Blanco et al./2005 [45] |
ACC (Ad) |
TN |
Neg. |
NA |
NA |
NA |
|
Abite et al./2005 [46] |
IDC |
NA |
NA |
NA |
NA |
NA |
|
Stafyla et al./2004 [47] |
LCIS |
NA |
NA |
24 |
No |
No |
|
Abe et al./ 2004 [48] |
IDC |
PR |
Pos. |
59 |
No |
No |
|
Adelekan et al./2003 [49] |
IC, LCIS - DCIS |
NA, NA |
NA, Pos. |
NA, NA |
NA |
No |
|
Yano et al./2001 [50] |
LCIS |
NA |
Neg. |
24 |
No |
No |
|
Gebrim et al./2000 [51] |
ILC |
NA |
Neg. |
NA |
No |
No |
|
Psarianos et al./1998 [52] |
DCIS |
NA |
NA |
NA |
NA |
NA |
|
Shah et al./ 1998 [53] |
LCIS |
NA |
NA |
25 |
No |
No |
|
Kurosum et al./1994 [54] |
IDC |
NA |
NA |
NA |
NA |
No |
|
Morimoto et al./1993 [55] |
LCIS |
NA |
NA |
132 |
No |
No |
|
Gupta et al./1992 [56] |
DCIS |
NA |
NA |
9 |
No |
No |
|
Gupta et al./1991 [57] |
CA |
NA |
Neg. |
10 |
No |
No |
|
Fukud et al./1989 [58] |
LCIS |
NA |
NA |
NA |
No |
No |
|
Yoshida et al./1985 [59] |
ILC |
ER |
Neg. |
32 |
No |
No |
|
Fond et al./1979 [60] |
DCIS |
NA |
Neg. |
NA |
NA |
NA |
|
Konakry et a./1975 [61] |
LCIS |
NA |
Neg. |
60 |
No |
No |
|
LCIS |
NA |
Neg. |
36 |
No |
No |
|
|
LCIS |
NA |
Neg. |
36 |
No |
No |
|
|
LCIS |
NA |
Neg. |
24 |
No |
No |
|
|
LCIS |
NA |
Neg. |
NA |
No |
No |
|
|
Durso et al./1972 [62] |
LCIS |
NA |
NA |
NA |
NA |
NA |
| NA: non-available, DCIS: Ductal Carcinoma In Situ, IDC: Invasive Ductal Carcinoma, CIS: Carcinoma In Situ, IAC: Invasive apocrine LCIS - DCIScarcinoma, ILC: Invasive Lobular Carcinoma, LCIS: Lobular Carcinoma In Suspicious, , ACC (ac): Acinic Cell Carcinoma, ACC (Ad): Adenoid Cystic Carcinoma, IC: Invasive Carcinoma, CA: Carcinoma, ER: Estrogen Receptor, PR: Progesterone Receptor, HER2: Human Epidermal Growth Factor Receptor 2, TN: Triple Negative, HPE: Histopathological Examination, Pos.: positive, Neg.: negative, FU: Follow-up. | ||||||
Patients and tumor characteristics
The total number of patients was 72 females, with a mean age of 44.4 ± 13.6 years. Most patients presented with either a breast lump (43.1%) or a mass (30.5%), with a median presentation duration of 12 months. In 80.6% of cases, the disease was unilateral, with laterality distributed almost equally between the right side (42.1%) and the left (39.5%). The mean tumor size was 24.7 ± 13.3 millimeters. The past medical history was negative in 27.8% of cases, while seven cases (9.7%) had a positive history of other breast diseases, including breast mass in four cases and DCIS, fibrocystic breast disease, and IDC per case. The family history of breast cancer was positive in four cases (5.5%). On physical examination, information about the tumor surface was available for nine tumors (11.8%), all of which had a smooth surface. Of the 22 tumors with available data on consistency, 14 (63.6%) were firm, five (22.7%) were hard, and three (13.6%) were rubbery. Among the 28 tumors with existing mobility data, 25 (89.3%) were found to be mobile. Axillary lymphadenopathy was reported in four tumors (5.3%). On ultrasonography, 28 masses appeared benign (36.8%), and 20 cases were suspicious (26.3%), with ultrasonographic data unavailable for the remaining tumors (36.8%). Mammography data were available for 50 tumors, revealing 27 benign lesions (54%) and 23 suspicious lesions (46%). Among the 17 lesions with available magnetic resonance imaging (MRI) data, five were benign lesions (29.4%), and 12 were suspicious (70.6%). Core needle biopsy (CNB) or fine needle aspiration cytology (FNAC) revealed that 20 tumors (26.3%) were benign, 24 (31.6%) were malignant, and two (2.6%) were suspicious. The data on preoperative diagnosis was unavailable for 30 cases (39.5%). (Table 7).
|
Variables |
Frequency/ percentages |
|
Study design Case report Case series |
58 (95.0%) 3 (5.0 %) |
|
Country Japan USA Korea Brazil China Italy Taiwan Australia India New Zealand Singapore Turkey Others |
12 (19.7%) 9 (14.7%) 4 (6.6%) 3 (4.9%) 3 (4.9%) 3 (4.9%) 3 (4.9%) 2 (3.3%) 2 (3.3%) 2 (3.3%) 2 (3.3%) 2 (3.3%) 14 (22.9%) |
|
Age range (mean ± SD) |
18-80 (44.4 ± 13.6) |
|
Gender Female |
72 (100%) |
|
Presentation Lump Mass Incidental Nodule Nipple discharge N/A |
31 (43.1%) 22 (30.5%) 3 (4.1%) 2 (2.8%) 1 (1.4%) 13 (18.1%) |
|
Duration of presentation, median (Q1 - Q3), months |
12 (2-60) |
|
Laterality Unilateral Bilateral N/A |
58 (80.6%) 6 (8.3%) 8 (11.1%) |
|
Tumor location Right Left Bilateral N/A |
32 (42.1%) 30 (39.5%) 1 (1.3%) 13 (17.1%) |
|
Tumor size (mean ± SD), mm |
24.7 ± 13.3 |
|
PMH Nothing noteworthy Breast mass Hypertension CAH DCIS Fibrocystic breast disease IDC N/A |
20 (27.8%) 4 (5.5%) 1 (1.4%) 1 (1.4%) 1 (1.4%) 1 (1.4%) 1 (1.4%) 43 (59.7%) |
|
Family history of breast cancer Positive Negative N/A |
4 (5.5%) 31 (43.1%) 37 (51.4%) |
|
Surface of the mass Smooth N/A |
9 (11.8%) 67 (88.2%) |
|
Consistency of the mass Firm Hard Rubbery N/A |
14 (18.4%) 5 (6.6%) 3 (3.9%) 54 (71.1%) |
|
Mobility of the mass Mobile Non-mobile Partially fixed N/A |
25 (32.9%) 2 (2.6%) 1 (1.3%) 48 (63.2%) |
|
Axillary Lymphadenopathy Negative Positive N/A |
27 (35.5%) 4 (5.3%) 45 (59.2%) |
|
Radiological findings |
|
|
Ultrasonography Benign Suspicious N/A |
28 (36.8%) 20 (26.3%) 28 (36.8%) |
|
Mammography Benign Suspicious N/A |
27 (35.5%) 23 (30.3%) 26 (34.2%) |
|
Magnetic resonance imaging Suspicious Benign N/A |
12 (15.8%) 5 (6.6%) 59 (77.6%) |
|
Shape of the mass Oval Irregular Lobular Round Multilobulated N/A |
15 (19.7%) 2 (2.6%) 2 (2.6%) 2 (2.6%) 1 (1.3%) 54 (71.1%) |
|
Margin of the mass Well defined Irregular Ill-defined Lobulated Smooth Microlobulated Circumscribed N/A |
32 (42.1%) 7 (9.2%) 4 (5.3%) 3 (4%) 2 (2.6%) 1 (1.3%) 1 (1.3%) 26 (34.2%) |
|
Vascularity of the mass Yes No N/A |
8 (10.5%) 2 (2.6%) 66 (86.8%) |
|
Calcification Positive Negative N/A |
24 (31.6%) 11 (14.5%) 41 (53.9%) |
|
Cytology (CNB or FNAC) Benign Malignant (non-specified) DCIS IDC IDC – DCIS Suspicious IC ILC – LCIS Invasive apocrine carcinoma N/A |
20 (26.3%) 8 (10.5%) 7 (9.2%) 3 (4%) 3 (4%) 2 (2.6%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 30 (39.5%) |
|
Breast surgery Wide local excision Mastectomy Excisional biopsy N/A |
37 (50.7%) 24 (32.9%) 9 (12.3%) 3 (4.1%) |
|
Axillary surgery ALND SLNB None N/A |
17 (23.3%) 15 (20.6%) 29 (39.7%) 12 (16.4%) |
|
Chemotherapy Yes No NA |
11 (15.3%) 15 (20.8%) 46 (63.9%) |
|
Radiation therapy Yes No NA |
16 (22.2%) 14 (19.4%) 42 (58.3%) |
|
Hormonal therapy Yes No NA |
20 (27.8%) 2 (2.8%) 50 (69.4%) |
|
Post-operative HPE DCIS LCIS IDC IDC - DCIS ILC ILC - LCIS Carcinoma (non-specified) Acinic cell carcinoma Adenoid cystic carcinoma IDC - ILC - DCIS Invasive apocrine carcinoma LCIS – DCIS N/A |
23 (30.3%) 15 (19.7%) 15 (19.7%) 6 (7.9%) 5 (6.6%) 3 (4%) 3 (4%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) |
|
Immunohistochemistry ER – PR Triple-negative ER HER2 ER - PR - HER2 PR - HER2 PR N/A |
19 (25%) 8 (10.5%) 6 (7.9%) 4 (5.3%) 1 (1.3%) 1 (1.3%) 1 (1.3%) 36 (47.4%) |
|
Axillary status Positive Negative N/A |
7 (9.2%) 32 (42.1%) 37 (48.7%) |
|
Follow-up, median (Q1-Q3), months |
24 (10-36) |
|
Recurrence No Yes N/A |
38 (52.8%) 1 (1.4%) 33 (45.8%) |
|
Metastasis No Yes N/A |
43 (59.7%) 2 (2.8%) 27 (37.5%) |
|
SD: Standard Deviation, N/A: non-available, CAH: Congenital Adrenal Hyperplasia, DCIS: Ductal Carcinoma In Situ, IDC: Invasive Ductal Carcinoma, CNB: Core Needle Biopsy, FNAC: Fine Needle Aspiration Cytology, CIS: Carcinoma In Situ, IC: Invasive Carcinoma, ILC: Invasive Lobular Carcinoma, LCIS: Lobular Carcinoma In Situ, ALND: Axillary Lymph Node Dissection, SLNB: Sentinel Lymph Node Biopsy, HPE: Histopathological Examination, ER: Estrogen Receptor, PR: Progesterone Receptor, HER2: Human Epidermal Growth Factor Receptor 2, Q1:first quartile, Q3: third quartile, PMH: past medical history. |
|
Management and outcome
The most commonly performed surgery was wide local excision (50.7%), followed by mastectomy (32.9%). Axillary lymph node dissection was carried out in 43.9% of cases. A total of 11 cases (15.3%) received chemotherapy, 16 cases (22.2%) underwent radiotherapy, and hormonal therapy was prescribed for 20 cases (27.8%). On histopathological examination, 11 tumors exhibited more than one pathology. DCIS was the most frequent finding (40.8%), followed by IDC (28.9%) and LCIS (28.4%). Immunohistochemical analysis showed that 20 out of 40 tumors (50%) were positive for both estrogen (ER) and progesterone receptors (PR). Of the 39 tumors with reported axillary status, 82.1% had negative axillary findings. The median follow-up period was 24 months, with quartile ranges of 10 to 36 months. Recurrence was observed in one case (1.4%), and metastasis occurred in two cases (2.8%) (Table 7).
Discussion
Carcinomas and high-risk lesions within fibroadenomas can either originate from the fibroadenoma itself and remain entirely encapsulated, or they can involve both the fibroadenoma and the adjacent breast tissue [2]. While rare, a small percentage of fibroadenomas may contain carcinomas or high-risk lesions, with reported incidence rates ranging from 0.002% to 0.125%. Fibroadenomas with malignant components are primarily found in patients 10 to 20 years older than the typical age for simple fibroadenomas [2]. In this systematic review, the mean age of affected patients was 44.4 years, further highlighting the trend of malignancies occurring in later decades of life.
The role of fibroadenomas as a potential risk factor for breast cancer is still not fully established [8]. It has been suggested that they may represent a long-term risk factor for breast cancer, particularly in women with complex fibroadenomas, proliferative disease, or a family history of breast cancer. Specifically, complex fibroadenomas are associated with a relative breast cancer risk that is approximately 3.10 times greater [6]. Another significant indicator of potential malignant transformation in fibroadenomas is the progressive mass size and thickness increase with advancing patient age [3]. A study has reported that the average tumor diameter of breast cancer occurring within a fibroadenoma is 2.46 cm [11]. Similarly, the mean tumor size in this systematic review was 2.47 ± 13.3 cm.
Frisch et al. reported that the predominant form of malignancy associated with breast cancer arising in fibroadenomas was CIS, with LCIS accounting for 66.9% and DCIS comprising 12.4%. Additionally, IDCs were more frequent among the invasive cases than ILCs [7]. Conversely, another study found that ductal and lobular carcinomas occur with equal frequency [6]. In this study, the distribution of malignancies within fibroadenomas revealed distinct differences from Frisch et al.’s study [7]. Notably, DCIS was the most frequent malignancy, accounting for 40.8% of tumors and LCIS represented 28.4% of tumors. The incidence of IDC was higher in this review at 28.9%, compared to 11% in the prior study [7]. Additionally, rarer malignancies like acinic cell carcinoma, adenoid cystic carcinoma, and invasive apocrine carcinoma were observed, suggesting a broader spectrum of tumor types associated with fibroadenomas than traditionally recognized.
The neoplastic proliferation of epithelial cells within the breast lobule characterizes LCIS. It is considered a precursor to ILC, similar to the relationship between DCIS and IDC. LCIS is now recognized as a general marker for breast cancer risk rather than a definitive pre-cancerous condition. It has been indicated that neoplasms within fibroadenomas behave similarly and have comparable prognoses to those occurring independently [9]. DCIS, also known as intraductal carcinoma, is a neoplasm that does not invade the basement membrane. This type of breast carcinoma develops within the ductal system, particularly in the terminal lobular duct unit. Although DCIS cannot metastasize and is considered non-lethal, its presence indicates an increased risk of developing invasive cancer if left untreated [8].
The preoperative diagnosis of malignant transformation within fibroadenoma is difficult and often necessitates surgical intervention for definitive confirmation [3]. This challenge stems from the overlap in clinical and radiological features between benign and malignant fibroadenomas, making it difficult to distinguish between the two preoperatively [4]. However, certain imaging characteristics can help identify carcinoma within fibroadenomas. Such malignancies tend to present with larger size, irregular shape, poorly defined margins, and abnormal calcifications, including linear, pleomorphic, or microcalcifications [12]. Sonographic evaluation of carcinomas within fibroadenomas typically reveals irregular lesions with indistinct borders. These tumors are often associated with marked hypoechoic shadowing, an echogenic halo, and distortion of surrounding tissue. Ultrasound is beneficial for tumor size assessment due to its high-resolution imaging capabilities. While mammography may reveal indistinct borders and microcalcifications, it is insufficient for diagnosing fibroadenomas with underlying carcinoma. Nonetheless, microcalcifications on mammography remain a valuable indicator of malignant transformation [3]. When calcifications are identified on mammography, ultrasound can be used to evaluate the invasiveness of the lesion and guide biopsy. Additionally, Doppler color imaging provides further insights into the internal vascularity of the tumor [13]. Dynamic MRI offers a reliable method for distinguishing malignant transformations from benign fibroadenomas by highlighting differences in vascularity. Benign fibroadenomas typically appear as round or oval masses with smooth margins on MRI, showing consistent enhancement into the late phase. In contrast, malignant lesions often display rapid early enhancement with variability in delayed enhancement, a hallmark of carcinoma [3]. Detecting malignant transformation can be particularly challenging, as clinical and radiological signs may remain masked until the tumor breaches the false capsule. As a result, definitive diagnosis is usually made through histopathological examination, emphasizing the importance of maintaining a high index of suspicion in these cases [3,4]. In the present study, of the 22 cases that reported tumor shape on imaging, 15 (68.2%) presented with an oval shape, while two cases (9.1%) showed an irregular shape. Tumor margins were well-defined in 32 out of 50 cases (64%), whereas seven (14%) exhibited irregular margins. Among the 10 cases reporting tumor vascularity, eight (80%) showed increased or high vascularity. Calcifications were observed in 24 out of 35 cases (68.6%) that provided data on this feature.
Common clinical techniques for obtaining pathological information include FNAC, hollow CNB, and mass excision biopsy. However, due to the inherent heterogeneity of these lesions, FNAC and CNB may not always provide conclusive results to definitively exclude malignancy in benign breast lesions that carry an increased risk of cancer development. Consequently, an open biopsy is recommended as a more reliable method for accurate diagnosis [15]. If imaging studies of a fibroadenoma indicate enlargement or any abnormal changes during follow-up examinations, it is essential to perform a CNB to ensure a definitive assessment. For patients aged 40 years and older with clinically benign fibroadenomas, clinicians should engage in discussions with these patients regarding the potential necessity of a CNB. This proactive approach allows for a thorough evaluation of changes and ensures appropriate diagnostic measures are implemented [12]. The diagnosis of fibroadenoma with carcinoma in the breast is contingent upon several critical criteria. Firstly, there must be clear evidence of epithelial heterogeneous hyperplasia or carcinoma within the fibroadenoma. Secondly, the cancerous tissue should remain confined to the capsule of the fibroadenoma, with only minimal focal infiltration into the surrounding breast tissue. Thirdly, it is crucial to exclude the possibility of infiltration from adjacent breast cancer into the fibroadenoma, as the coexistence of breast cancer and fibroadenoma does not qualify as intra-fibroadenoma carcinoma. Finally, the diagnosis must be supported by the results of immunohistochemical markers. These criteria facilitate a thorough and accurate assessment of fibroadenoma with carcinoma [15]. In this systematic review, pre-operative tissue biopsy using either CNB or FNAC was available for 46 tumors. Malignant features were observed in 24 tumors (52.2%), two tumors (4.3%) exhibited suspicious features, and 20 tumors (43.5%) were classified as benign. These findings highlight the importance of pre-operative biopsy and the challenges in accurately identifying the presence of malignancy in fibroadenomas.
Given the rarity of malignancy arising within fibroadenomas, standardized management guidelines are not well-established, leaving uncertainty as to whether these patients should be treated similarly to breast cancer patients or with a distinct approach. For benign fibroadenomas, lumpectomy remains the treatment of choice. However, if the tumor is close to or involves the resection margin, wider local excision may be necessary to ensure complete removal. Factors such as large tumor size, multifocality, and central breast location may also necessitate consideration of mastectomy [3,4,16]. If surgical margins are free of cancer, lumpectomy alone is often sufficient. The overall management strategy is dictated by the stage of the disease and the degree of metastasis, whether localized or distant. Conservative management, such as lumpectomy or wide local excision, is usually appropriate for small tumors. In cases of local metastasis, especially involving the axillary lymph nodes, axillary lymph node dissection is typically performed to ensure proper treatment [3]. Surgical intervention remains the definitive treatment and may be combined with radiotherapy or chemotherapy depending on individual case specifics [16]. In the current study, the most common procedure was wide local excision (50.7%), followed by mastectomy (32.9%). Excisional biopsy was performed in 12.3% of the cases. Axillary lymph node dissection was performed in 17 cases (23.3%), while sentinel lymph node biopsy was carried out in 15 cases (20.6%). Twenty-nine cases (39.7%) did not undergo axillary surgery. This variation in axillary management highlights the individualized approach to surgical treatment based on tumor characteristics, lymph node involvement, and disease progression.
The use of radiotherapy remains a topic of debate, with chemotherapy being the preferred treatment option in cases involving nodal metastasis. Some authors suggested that breast cancer arising within a fibroadenoma exhibits similar behavior to breast cancer at the same stage. Consequently, the treatment approach should align with standard breast cancer protocols, following similar therapeutic modalities [4,5,11,17]. The positive impact of radiation therapy on both survival rates and recurrence prevention when combined with lumpectomy has been reported. This approach is regarded as the standard of care for breast-conserving therapy in cases of DCIS and breast cancer. However, radiation therapy is not without drawbacks. It carries inherent risks, financial costs, and potential negative effects on patients' quality of life. Notably, long-term complications such as lung cancer and heart disease have been associated with breast cancer radiation therapy, particularly in patients who have a history of smoking [17]. Ni et al. stated that DCIS within a fibroadenoma is a heterogeneous condition with significant variability in local recurrence risks among patients. Consequently, the overall benefits of postoperative radiation therapy differ based on individual patient risk profiles. Low-risk patients who undergo breast-conserving surgery (BCS) without subsequent radiotherapy experience limited advantages from radiation. In contrast, high-risk patients show a greater benefit from the addition of radiotherapy. For instance, it has been revealed that patients treated with BCS alone had 8-year recurrence rates of 0%, 21.5%, and 32.1% for low-, intermediate-, and high-risk groups, respectively. This highlights the need for personalized treatment approaches based on risk stratification [15]. The current National Comprehensive Cancer Network (NCCN) guidelines recommend ER testing for patients with DCIS and advise considering tamoxifen for women with ER-positive disease, particularly those who undergo BCS without radiation. The goal is to optimize treatment outcomes and minimize the chances of cancer recurrence [7]. In this study, the data on chemotherapy was available for only 26 cases, of which 11 (42.3%) underwent chemotherapy as part of their treatment regimen. Additionally, among 30 cases with information on radiation therapy, 16 cases (53.3%) received the treatment regimen. Furthermore, 22 cases addressed hormonal therapy, and 20 (90.9%) indicated it was utilized in the treatment protocols.
Some scholars indicated that breast cancer developing within a fibroadenoma is generally associated with a more favorable prognosis compared to conventional breast cancer. This is primarily attributed to the higher incidence of hormone receptor (HR)-positive tumors in this subset, along with the frequent presentation of CIS and early-stage disease at diagnosis [12]. However, the prevalence of hormone receptor positivity in these cases may not significantly differ from that seen in typical breast cancer. ER positivity has been reported at 68.8%, and PR positivity at 62.5%, figures closely aligned with those observed in conventional breast cancer [11]. Despite these favorable characteristics, it has been indicated that approximately 10% of patients diagnosed with CIS within a fibroadenoma experience recurrence or metastasis, emphasizing the need for continued surveillance and individualized treatment strategies, even in cases with seemingly better prognostic indicators [3]. In this systematic review, among the 40 tumors with available hormone receptor status, six (15%) were HR-positive. The ER was positive in 26 tumors (65%), and PR was positive in 22 tumors (55%). The median follow-up duration was 24 months, during which one case (1.4%) reported recurrence, and two cases (2.8%) experienced metastasis. The primary limitation of this study is the lack of data on several variables in the reviewed studies, which may impact the generalizability of the findings.
Conclusion
Although rare, carcinomas arising within fibroadenomas may present considerable challenges in preoperative diagnosis, whether through imaging or cytology. Therefore, clinicians may find it necessary to approach fibroadenomas with increased caution.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: AMS, LRAP and SL were significant contributors to the conception of the study and the literature search for related studies. BAA, DAH, BOH, HAH, SHS, DAO, SSA and SMA were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. MGH, MNH and HOA were involved in the literature review, study design, and manuscript writing. YMM, HAH and SSO Literature review, final approval of the manuscript, and processing of the tables. HOA and AMS confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
References
- Tagliati C, Lanni G, Cerimele F, et al. Low diffusion level within a fibroadenoma as the sole sign of ductal carcinoma in situ: A case report. Breast Disease. 2021;40(4):283-6. doi:10.3233/bd-201080
- Pang FT, Kornecki A, Lynn K, et al. A rare case of lobular carcinoma in situ within a fibroadenoma. Arch Breast Cancer. 2022;119-22. doi:10.32768/abc.202291119-122
- Hammood ZD, Mohammed SH, Abdulla BA, et al. Ductal carcinoma in situ arising from fibroadenoma; a rare case with review of literature. Ann Med Surg. 2022;75:103-8. doi:10.1016/j.amsu.2022.103449
- Brunetti N, Valente I, Pitto F, et al. Triple-negative breast cancer arising in a fibroadenoma in BRCA 1 mutated patient. J Med Ultrasound. 2023;31(1):63-5. doi:10.4103/jmu.jmu_83_21
- Wang M, Leong MY, Tan QT. A case of fibroadenoma with lobular carcinoma in situ and microinvasion in a young woman. J Surg Case Rep. 2022;2022(12). doi: N/A
- Shojaku H, Hori R, Yoshida T, et al. Low-grade ductal carcinoma in situ (DCIS) arising in a fibroadenoma of the breast during 5 years follow-up: a case report. Medicine. 2021;100(10). doi:10.1097/md.0000000000024023
- Frisch O, De Jager LJ, Otto M, et al. A teenager with ductal carcinoma in situ arising in a fibroadenoma: a case report. S Afr J Surg. 2018;56(4):30-3. doi:10.17159/2078-5151/2018/v56n4a2766
- Feijó CQ, da Costa RE, da Silva Fontinele DR, et al. Low-grade carcinoma in situ in fibroadenoma: a case report. Mastology. 2021;31:1-3. doi:10.29289/2594539420210058
- Brock CM, Harper C, Tyler T. Fibroadenoma containing lobular carcinoma in situ, an unusual finding in a normally benign mass. J Surg Case Rep. 2020;2020(4). doi:10.1093/jscr/rjaa059
- Abdullah HO, Abdalla BA, Kakamad FH, et al. Predatory publishing lists: A review on the ongoing battle against fraudulent actions. Barw Med J. 2024;1(1):45-9. doi:10.58742/bmj.v2i2.91
- Fujimoto A, Matsuura K, Kawasaki T, et al. Early HER2-positive breast cancer arising from a fibroadenoma: a case report. Oxf Med Case Rep. 2021;2021(9). doi:10.1093/omcr/omab083
- Shiino S, Yoshida M, Tokura M, et al. Locally advanced triple-negative breast cancer arising from fibroadenoma with complete response to neoadjuvant chemotherapy: a case report. Int J Surg Case Rep. 2020;68:234-8. doi:10.1016/j.ijscr.2020.02.059
- Gonthong A. Ductal carcinoma in situ within fibroadenoma: report of imaging features. J Dep Med Serv. 2021;46(2):151-8. doi: N/A
- Ni XH, An R, Shi QW, et al. Low-grade ductal carcinoma in situ within a fibroadenoma of the breast: A rare case report and review of the literature. OncoTargets Ther. 2023;16:399-406. doi:10.2147/ott.s413223
- Tiwari A, Singh BM, Varshney S, et al. Incidental detection of carcinoma in situ in fibroadenoma of the breast in a young woman: a rare finding. Cureus. 2018;10(12). doi:10.7759/cureus.3797
- Marumoto A, Steinemann S, Furumoto N, et al. An uncommon pairing of common tumors: case report of ductal carcinoma in situ within fibroadenoma. Hawai'i J Med Public Health. 2019;78(2):39. doi:10.5348/100916z01sz2018cr
- Moreno M, Menegassi J, Neto OV, et al. Metachronous breast neoplasms: squamous cell carcinoma and lobular carcinoma in situ within a fibroadenoma. Mastology. 2020;30:1-4. doi:10.29289/25945394202020200016
- El-Essawy M, AL Haidary A, Khan AL. Ductal carcinoma in situ (DCIS) in breast fibroadenoma. Egypt J Radiol Nucl Med. 2020;51:1-7. doi:10.1186/s43055-020-00191-5
- Zeeshan S, Siddique S, Riaz N. Ductal carcinoma in situ within fibroadenoma of the breast: a case report with literature review. Int J Case Rep Images. 2018;9:100916Z01SZ2018. doi:10.5348/100916Z01SZ2018CR
- Lim S, Shim MK, Cho EY, et al. Secretory carcinoma arising in a fibroadenoma: a brief case report. J Pathol Transl Med. 2018;52(3):198-201. doi:10.4132/jptm.2017.08.01
- You JK, Kim YJ, Kim B, et al. Ductal carcinoma in situ within a fibroadenoma: microcalcifications identified on mammography play a crucial role in diagnosis. J Korean Soc Radiol. 2016;74(6):361-4. doi:10.3348/jksr.2016.74.6.361
- Zheng H, Chen J, Wu X, et al. Bilateral breast cancer with a unilateral carcinoma within a fibroadenoma: a case report. Oncol Lett. 2015;10(3):1513-6. doi:10.3892/ol.2015.3420
- Hua B, Xu JY, Jiang L, et al. Fibroadenoma with an unexpected lobular carcinoma in situ: a case report and review of the literature. Oncol Lett. 2015;10(3):1397-401. doi:10.3892/ol.2015.3488
- Wu YT, Chen ST, Chen CJ, et al. Breast cancer arising within fibroadenoma: collective analysis of case reports in the literature and hints on treatment policy. World J Surg Oncol. 2014;12:335. doi:10.1186/1477-7819-12-335
- Mele M, Vahl P, Funder JA, et al. Apocrine carcinoma arising in a complex fibroadenoma: a case report. Breast Dis. 2014;34(4):183-7. doi:10.3233/bd-140365
- Limite G, Di Micco R, Esposito E, et al. The first case of acinic cell carcinoma of the breast within a fibroadenoma: case report. Int J Surg. 2014;12. doi:10.1016/j.ijsu.2014.05.005
- Kwon MJ, Park HR, Seo J, et al. Simultaneous occurrence of ductal carcinoma in situ within juvenile fibroadenoma in both breasts: a brief case report. Korean J Pathol. 2014;48(2):164. doi:10.4132/koreanjpathol.2014.48.2.164
- Kılıç F, Ustabaşıoğlu FE, Samancı C, et al. Ductal carcinoma in situ detected by shear wave elastography within a fibroadenoma. J Breast Cancer. 2014;17(2):180. doi:10.4048/jbc.2014.17.2.180
- Dandin Ö, Karakaş DÖ, Balta AZ, et al. Invasive breast carcinoma occurring within fibroadenoma: case report. Turkiye Klinikleri J Case Rep. 2015;23(2):149-52. doi:10.5336/caserep.2013-37780
- Buteau AH, Waters RE, Boroumand N. Metastatic carcinoma arising from fibroadenoma of the breast. Int J Cancer Oncol. 2014;1:1-3. doi: N/A
- Hayes BD, Quinn CM. Microinvasive lobular carcinoma arising in a fibroadenoma. Int J Surg Pathol. 2013;21(4):419-21. doi:10.1177/1066896912471854
- Jahan F, Ghosh PK, Rahman S, et al. Fibroadenoma with foci of infiltrating ductal carcinoma. J Med. 2012;13(1):115. doi:10.3329/jom.v13i1.10088
- Butler R, Pinsky R, Jorns JM, et al. Alveolar variant of invasive lobular carcinoma in a fibroadenoma. Breast J. 2012;18:645-50. doi:10.1111/tbj.12016
- Ooe A, Takahara S, Sumiyoshi K, et al. Preoperative diagnosis of ductal carcinoma in situ arising within a mammary fibroadenoma: a case report. J Clin Oncol. 2011;41:918-23. doi:10.1093/jjco/hyr075
- Lin IL, Lee YH, Kao WS, et al. Pre-operative diagnosis of invasive ductal carcinoma within a fibroadenoma by mammography. J Radiol Sci. 2011;36:28-31. doi:10.1097/pcr.0000000000000478
- Kato F, Omatsu T, Matsumura W, et al. Dynamic MR findings of ductal carcinoma in situ within a fibroadenoma. Magn Reson Med Sci. 2011;10:129-32. doi:10.2463/mrms.10.129
- Abu-Rahmeh Z, Nseir W, Naroditzky I, et al. Invasive ductal carcinoma within fibroadenoma and lung metastases. Int J Gen Med. 2012;5:19-21. doi:10.2147/ijgm.s26115
- Rao S, Latha PS, Ravi A, et al. Ductal carcinoma in a multiple fibroadenoma: diagnostic inaccuracies. J Cancer Res Ther. 2010;6:385-7. doi:10.4103/0973-1482.73350
- Petersson F, Tan PH, Putti CT, et al. Low-grade ductal carcinoma in situ and invasive mammary carcinoma with columnar cell morphology arising in a complex fibroadenoma in continuity with columnar cell change and flat epithelial atypia. Int J Surg Pathol. 2010;18:352-7. doi:10.1177/1066896910373096
- Tajima S, Kanemaki Y, Kurihara Y, et al. A case of a fibroadenoma coexisting with an invasive lobular carcinoma in the breast. Breast Cancer. 2011;18:319-23. doi:10.1007/s12282-009-0122-z
- Gashi-Luci LH, Limani RA, Kurshumliu FI, et al. Invasive ductal carcinoma within fibroadenoma: a case report. Cases J. 2009;2:1-4. doi:10.1186/1757-1626-2-174
- Borecky N, Rickard M, et al. Preoperative diagnosis of carcinoma within fibroadenoma on screening mammograms. J Med Imaging Radiat Oncol. 2008;52:64-7. doi:10.1111/j.1440-1673.2007.01913.x
- Tiu CM, Chou YH, Chiou SY, et al. Development of a carcinoma in situ in a fibroadenoma: color Doppler sonographic demonstration. J Ultrasound Med. 2006;25:1335-8. doi:10.7863/jum.2006.25.10.1335
- Shin JH, Choi HY, Lee SN, et al. Microinvasive ductal carcinoma arising within a fibroadenoma: a case report. Acta Radiol. 2006;47:643-5. doi:10.1080/02841850600698838
- Blanco M, Egozi L, Lubin D, et al. Adenoid cystic carcinoma arising in a fibroadenoma. Ann Diagn Pathol. 2005;9:157-9. doi:10.1016/j.anndiagpath.2005.02.008
- Gogo-Abite M, Seleye-Fubara D, Jamabo RS, et al. Fibroadenoma coexisting with infiltrating ductal carcinoma: a case report. Niger J Med. 2005;14:221-3. doi:10.4314/njm.v14i2.37185
- Stafyla V, Kotsifopoulos N, GrigoriC ades K, et al. Lobular carcinoma in situ of the breast within a fibroadenoma: a case report. Gynecol Oncol. 2004;94:572-4. doi:10.1016/j.ygyno.2004.04.031
- Abe H, Hanasawa K, Naitoh H, et al. Invasive ductal carcinoma within a fibroadenoma of the breast. Int J Clin Oncol. 2004;9:334-8. doi:10.1007/s10147-004-0401-9
- Adelekan MO, Tresadern JC, Prescott R, et al. Multiple metastatic deposits of a left breast cancer into a right fibroadenoma. Breast. 2003;12:294-6. doi:10.1016/s0960-9776(03)00097-3
- Yano Y, Ueno E, Kamma H, et al. Non-invasive lobular carcinoma within a fibroadenoma: a preoperatively diagnosed case. Breast Cancer. 2001;8:70-3. doi:10.1007/bf02967481
- Gebrim LH, Bernardes Júnior JR, Nazário AC, et al. Malignant phyllodes tumor in the right breast and invasive lobular carcinoma within fibroadenoma in the other: case report. Sao Paulo Med J. 2000;118:46-8. doi:10.1590/s1516-31802000000200004
- Psarianos T, Kench JG, Ung OA, et al. Breast carcinoma in a fibroadenoma: diagnosis by fine needle aspiration cytology. Pathology. 1998;30:419-21. doi:10.1080/00313029800169736
- Shah A, Pathak R, Banerjee S, et al. Lobular carcinoma-in-situ within a fibroadenoma of the breast. Postgrad Med J. 1999;75:293. doi:10.1136/pgmj.75.883.293
- Kurosumi M, Itokazu R, Mamiya Y, et al. Invasive ductal carcinoma with a predominant intraductal component arising in a fibroadenoma of the breast. Pathol Int. 1994;44:874-7. doi:10.1111/j.1440-1827.1994.tb01687.x
- Morimoto T, Tanaka T, Komaki K, et al. The coexistence of lobular carcinoma in a fibroadenoma with a malignant phyllodes tumor in the opposite breast: report of a case. Surg Today. 1993; 23:656-60. doi:10.1007/bf00311918
- Gupta RK, Dowle C, et al. Fine needle aspiration of breast carcinoma in a fibroadenoma. Cytopathology. 1992; 3:49-53. doi:10.1111/j.1365-2303.1992.tb00022.x
- Gupta RK, Simpson J, et al. Carcinoma of the breast in a fibroadenoma: diagnosis by fine‐needle aspiration cytology. Diagn Cytopathol. 1991; 7:60-2. doi:10.1002/dc.2840070116
- Fukuda M, Nagao K, Nishimura R, et al. Carcinoma arising in fibroadenoma of the breast—A case report and review of the literature—. Jpn J Surg. 1989; 19:593-6. doi:10.1007/bf02471669
- Yoshida Y, Takaoka M, Fukumoto M, et al. Carcinoma arising in fibroadenoma: case report and review of the world literature. J Surg Oncol. 1985; 29:132-40. doi:10.1002/jso.2930290213
- Fondo EY, Rosen PP, Fracchia AA, et al. The problem of carcinoma developing in a fibroadenoma. Cancer. 1979; 43:563-7. doi:10.1002/1097-0142(197902)43:2%3C563::AID-CNCR2820430224%3E3.0.CO;2-H
- Buzanowski‐Konakry K, Harrison EG Jr, Payne WS, et al. Lobular carcinoma arising in fibroadenoma of the breast. Cancer. 1975; 35:450-6. doi:10.1002/1097-0142(197502)35:2%3C450::AID-CNCR2820350223%3E3.0.CO;2-R
- Durso EA, et al. Carcinoma arising in a fibroadenoma: a case report. Radiology. 1972; 102:565. doi:10.1148/102.3.565

This work is licensed under a Creative Commons Attribution 4.0 International License.

