Evolution of Antimicrobial Resistance in Community vs. Hospital-Acquired Infections
Abstract
Introduction
Hospitals are high-risk environments for infections. Despite the global recognition of these pathogens, few studies compare microorganisms from community-acquired and hospital-acquired infections (HAIs). This study compares these microorganisms and explores their relationship with patients' comorbidities and socio-demographic factors.
Methods
This retrospective cross-sectional study was conducted at Smart Health Tower, Iraq, from January to December 2023, focusing on patients with community-acquired infections and HAIs. Data were extracted from microbiology laboratory records, including blood cultures, urine samples, and other body fluids, with patients classified based on CDC and IDSA guidelines. Bacterial identification combined conventional methods and the BD Phoenix™ M50 system, while antibiotic susceptibility was tested using the Kirby-Bauer method and the same automated system. Statistical analysis of resistance patterns utilized SPSS version 25, with significance set at p ≤ 0.05.
Results
In this study of 2,157 participants, 1,303 (60.4%) were male, with microbial growth observed in 1,177 cases (54.6%). Notably, 41.1% of females and 52.1% of males showed no growth (p < 0.001). The mean age was 43.62 ± 23.3 years. Wound samples had the highest growth rate (91.2%), while body fluids had the lowest (33.0%) (p < 0.001). The overall multidrug resistance rates were 62.5% for community-acquired infections and 79.3% for HAIs. Patients with pre-existing comorbidities demonstrated significantly higher rates of hospital-acquired infections (p < 0.05).
Conclusion
Multidrug-resistant isolates are more prevalent in HAIs than in community-acquired infections, highlighting the need for enhanced surveillance to optimize antibiotic use and control HAIs through early detection of resistance.
Introduction
Hospitals represent a potentially hazardous environment due to various virulent pathogens introduced by admitted patients from the community. These patients are subsequently exposed not only to the hospital's endemic flora but also to microorganisms carried by other ill individuals [1]. This occurs due to a compromised immune defense and colonization by resistant organisms [2]. Hospital-acquired infections (HAIs) are a frequent occurrence in healthcare facilities globally, with their prevalence exceptionally high in resource-limited developing countries [3]. The extensive use of broad-spectrum antibiotics in hospitals creates an intense selective pressure, fostering the emergence of antibiotic-resistant bacteria and complicating the treatment of these infections. As a result, HAIs have been recognized as a severe public health issue for over a century, contributing to poor health outcomes and significantly impacting the quality of healthcare delivery [4].
Hospital-acquired infections most commonly manifest as urinary tract infections, respiratory tract infections, circulatory system infections, and surgical site infections [5]. A World Health Organization report covering 55 hospitals across 14 countries found that 8.7% of hospitalized patients developed HAIs, with the highest prevalence observed in the Eastern Mediterranean Region and lower rates in the Western Pacific [5]. The prevalence of HAIs has been reported at approximately 5% in North America and parts of Europe while reaching up to 40% in some areas of Asia, Latin America, and Africa [6]. A European study reported the prevalence of HAIs to be approximately 2.9%. Several factors contribute to the occurrence of HAIs, including medical interventions, substandard hospital environments, and inadequate personal hygiene practices among both hospital staff and patients [7]. However, the primary driver of HAIs is the failure to adhere to health and safety protocols in healthcare settings. While it is impossible to eliminate HAIs, even in highly advanced hospitals, strict adherence to established standards and guidelines can significantly reduce or manage their occurrence, especially in regions such as Africa [6]. In modern healthcare, where technological advancements and high expectations for quality care prevail, it is critical to thoroughly examine the frequency and underlying causes of HAIs. The absence of accurate data on the prevalence of HAIs poses significant challenges to executing these control measures, leading to increased healthcare costs for both health systems and patients [8].
Despite the global recognition of these pathogens, limited studies have compared microorganisms from both community and hospital settings; therefore, the current study aims to fill this gap by comparing microorganisms isolated from community-acquired and HAIs. It also seeks to explore the relationship between these infections and patients' comorbidities and socio-demographic factors.
Methods
Study design and setting
This retrospective cross-sectional study was conducted at Smart Health Tower, Iraq, between January 2023 and December 2023. It included patients from various departments of the hospital, with infections categorized as either community-acquired or HAIs. The Kscien Organization approved the study for Ethical Approval, reference number 24/No. 27, ensuring all ethical guidelines were followed throughout the study.
Sample collection and study population
Data were meticulously extracted from the records of patients who had their samples processed in the microbiology laboratory. Inclusion criteria encompassed all available clinical samples, including blood cultures, urine samples, sputum and bronchoalveolar lavage, wound swabs, and other body fluids. Patients were classified into either the CAI or HAI group based on guidelines from the Centers for Disease Control and Prevention and the Infectious Diseases Society of America. The CAIs were defined as infections present at the time of hospital admission or within 48 hours of admission, with no history of recent healthcare exposure, such as hospitalization within the previous 90 days. In contrast, HAIs were defined as infections that developed 48 hours or more after hospital admission and were associated with invasive procedures or prior healthcare exposure [9]. Patients with incomplete data were excluded to ensure the accuracy and reliability of the study findings.
Bacterial identification
Bacterial identification was conducted using conventional methods and the BD Phoenix™ M50 automated identification and susceptibility testing system, specifically tailored to the diverse range of clinical samples processed during the study. Blood cultures were incubated in the BD BACTEC™ automated blood culture system, following established protocols, for up to five days to detect the growth of bacteria or fungi, with positive cultures subsequently sub-cultured onto solid media, including blood agar and chocolate agar, to enhance isolation of pathogens. Urine samples were plated on cystine lactose electrolyte-deficient agar and MacConkey agar to promote the growth of Escherichia coli, Klebsiella, and other common uropathogens. Body fluids were inoculated onto blood and chocolate agar. To identify respiratory pathogens, sputum samples were Gram-stained and cultured on selective media, including MacConkey and blood agar. Wound swabs were processed on blood agar and mannitol salt agar. The BD Phoenix™ M50 system was utilized for precise species-level identification and antimicrobial susceptibility testing, providing comprehensive biochemical profiles for various pathogens [10]. This combination of conventional and automated methods ensured accurate identification and susceptibility testing across all clinical sample types, adhering to CLSI (Clinical and Laboratory Standards Institute) guidelines for bacteriological analysis [11]. For samples that did not exhibit visible growth after the initial 24 hours, the incubation was extended to 48 hours.
Antibiotic susceptibility testing
Antimicrobial susceptibility testing was conducted using two methods. The classical Kirby-Bauer disk diffusion method was performed according to Clinical and Laboratory Standards Institute guidelines [11], where standardized antibiotic disks were applied to Mueller-Hinton agar plates inoculated with bacterial suspensions, and inhibition zone diameters were measured and interpreted using CLSI breakpoints (Figure 1). Additionally, the BD Phoenix™ M50 automated system was used to confirm susceptibility results and to test a broader range of antimicrobials, providing Minimum Inhibitory Concentration (MIC) values and classifying isolates as susceptible, intermediate, or resistant based on CLSI interpretive criteria. The antibiotics tested included Amikacin, Gentamicin, Gentamicin-Syn, Ampicillin-sulbactam, Ampicillin, Amoxicillin, Amoxicillin-Clavulanate, Piperacillin-Tazobactam, Piperacillin, Penicillin G, Oxacillin, Cefuroxime, Ceftriaxone, Cefepime, Cefoxitin, Ceftaroline, Cefpodoxime, Cefixime, Cefotaxime, Clarithromycin, Azithromycin, Erythromycin, Ciprofloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, Ofloxacin, Trimethoprim-Sulfamethoxazole, Vancomycin, Teicoplanin, Daptomycin, Clindamycin, Tetracycline, Doxycycline, Minocycline, Tigecycline, Imipenem, Meropenem, Nitrofurantoin, Linezolid, Rifampin, Chloramphenicol, Mupirocin High level. This combined approach ensured consistent and accurate interpretation of susceptibility results, enhancing the reliability of the findings.
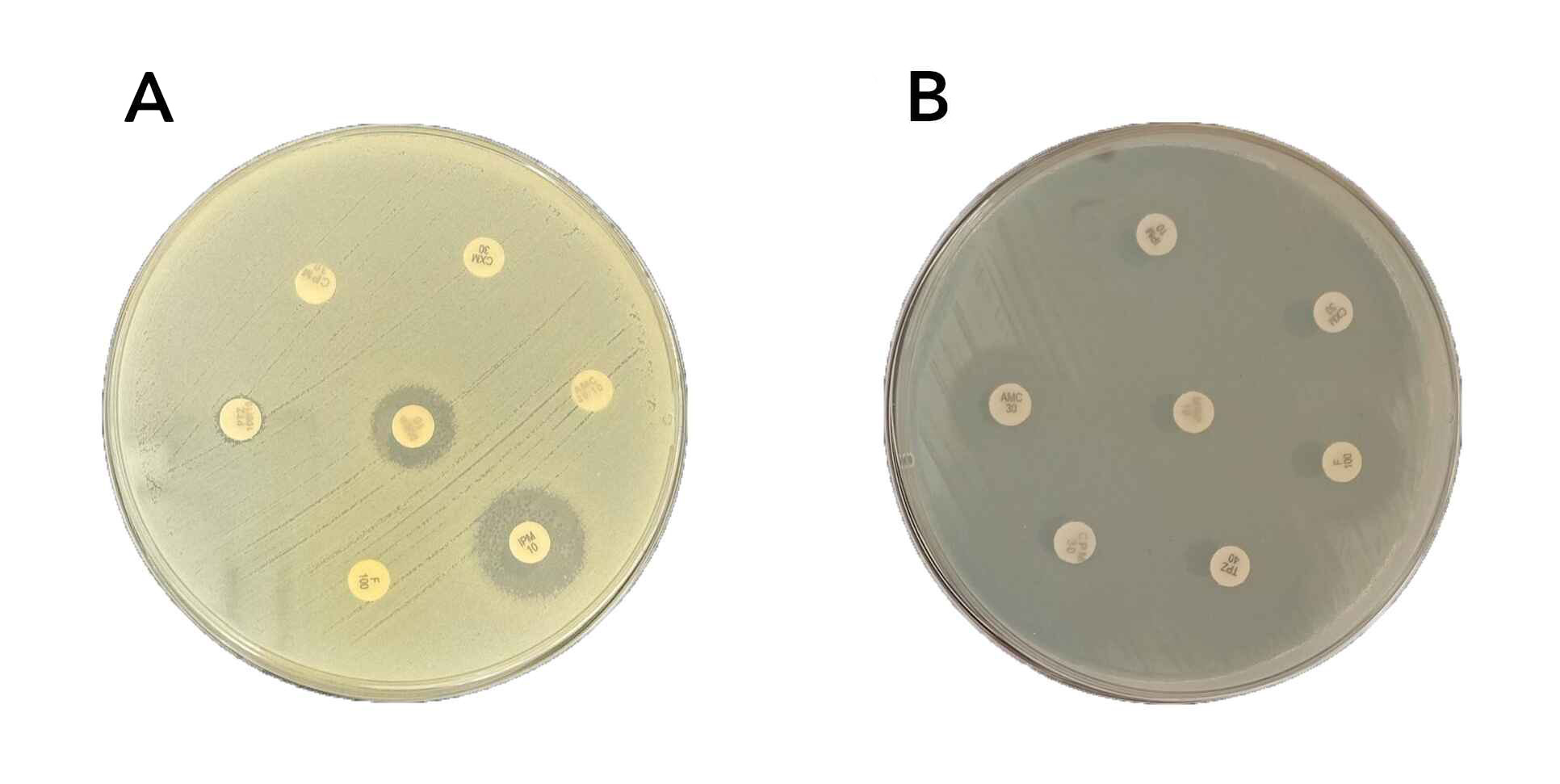
Antibiotic classification and multidrug resistance
The antibiotics were categorized into seven groups: aminoglycosides, beta-lactams, macrolides, sulfonamides, tetracyclines, glycopeptides, and fluoroquinolones. Multidrug-resistant (MDR) isolates were defined as bacterial strains resistant to three or more of these antibiotic classes, following established criteria [12]. This classification facilitated a comprehensive analysis of antimicrobial resistance patterns and enabled the identification of the most challenging cases of antibiotic resistance, providing critical insight into the prevalence of MDR organisms.
Data analysis
Data on bacterial isolates, antimicrobial susceptibility profiles, patient demographics, infection types, and antibiotic resistance patterns were systematically collected and entered into Microsoft Excel 2007 before being transferred to SPSS version 25 for statistical analysis. Statistical evaluations were conducted to assess differences in resistance rates between CAIs and HAIs, stratified by infection site (e.g., bloodstream, urinary tract, respiratory tract) and pathogen type. Descriptive statistics summarized the demographic and clinical characteristics of patients, while resistance rates were compared using Chi-square tests for categorical variables and t-tests for continuous variables. The analysis encompassed calculating prevalence rates, frequencies, susceptibility patterns, and other descriptive statistics, with statistical significance set at a p-value of equal to or less than 0.05 for the chi-square test, which compared categorical variables with bacterial growth.
Results
Microbial growth and participant characteristics
In this study involving 2157 participants, 1303 (60.4%) were male. Microbial growth was observed in 1177 cases (54.6%). Notably, 535 (41.1%) of the females and 445 (52.1%) of the males exhibited no growth, indicating a significant difference (p < 0.001). The mean age of participants was 43.62± 23.3years. The highest growth rate was observed in wound samples (187, 91.2%), while body fluids showed the lowest rate (171, 33.0%), reflecting a statistically significant difference (p < 0.001). The sample collection location did not significantly influence growth, with no growth in 475 (44.7%) from community settings and 216 (46.7%) from hospitals (p = 0.502). Among the various comorbidities, obesity, renal insufficiency, and diabetes, significantly differed between participants with microbial growth and those without growth(P<0.05) (Table 1).
|
Variables |
Bacterial Growth |
Total |
P-Value |
|
|
No Growth |
Growth |
|||
|
Gender (N, %) Female Male |
535(41.1) 445(52.1) |
768(58.9) 409(47.9) |
1303 (100) 854 (100) |
|
|
Age (Year, Mean± SD) |
43.38± 23.5 |
43.83± 23.2 |
43.62± 23.3 |
0.653 |
|
Type of clinical sample (N, %) Urine Body fluids Respiratory samples Wound Stool Pus Others |
534(45.1) 347(67.0) 32(35.6) 18(8.8) 8(42.1) 8(30.8) 33(28.9) |
651(54.9) 171(33.0) 58(64.4) 187(91.2) 11(57.9) 18(69.2) 81(71.1) |
1185(100) 518(100) 90(100) 205(100) 19(100) 26(100) 114(100) |
<0.001 |
|
Setting (N, %) Community Hospital Not mentioned |
216(46.7) 289(55.0) |
587(55.3) 247(53.3) 343(45.0) |
1062(100) 463(100) 632(100) |
0.502 |
|
Length of hospital stay (Day, Mean± SD) |
12.76± 27.72 |
9.42± 19.96 |
10.99± 23.95 |
0.137 |
|
Asthma (N, %) Yes No Not mentioned |
664(44.8) 290(46.2) |
22(45.8) 817(55.2) 338(53.8) |
48(100) 1482(100) 628(100) |
0.400 |
|
Pregnancy (N, %) Yes No Not mentioned |
24(43.6) 650(46.0) 306(44.5) |
31(53.4) 764(54.0) 382(55.5) |
55(100) 1414(100) 625(100) |
0.783 |
|
Heart Failure (N, %) Yes No Not mentioned |
96(49.2) 597(44.7) 287(45.9) |
99(50.8) 740(55.3) 338(54.1) |
195(100) 1337(100) 625(100) |
0.467 |
|
Renal insufficiency (N, %) Yes No Not mentioned |
67(35.4) 626(46.6) 287(45.9) |
122(64.6) 717(53.4) 338(54.1) |
189(100) 1343(100) 625(100) |
0.015 |
|
Hypertension (N, %) Yes No Not mentioned |
139(41.9) 554(46.2) 287(45.9) |
193(58.1) 646(53.8) 338(54.1) |
332(100) 1200(100) 625(100) |
0.364 |
|
Obesity (N, %) Yes No Not mentioned |
101(37.3) 591(46.9) 288(46.0) |
170(62.7) 669 (53.1) 338(54.0) |
271(100) 1260(100) 626(100) |
0.014 |
|
Malignant (N, %) No Not mentioned |
52(41.6) 641(45.6) 287(45.9) |
73(58.4) 766(54.4) 338(54.1) |
125(100) 1407(100) 625(100) |
0.667 |
|
Diabetes (N, %) Yes No Not mentioned |
103(35.4) 590(47.5) 287(46.0) |
188(64.6) 652(52.5) 337(54.0) |
291(100) 1242(100) 624(100) |
0.001 |
Distribution of isolated bacteria by setting
In this study, among the 449-gram negative bacterial isolates, 301 (67.0%) were from community settings, and 148 (33.0%) were from hospitals. Escherichia coli was the most prevalent, with 245 isolates, 179 (73.1%) from community settings and 66 (26.9%) from hospitals. Other notable gram-negative bacteria included Klebsiella pneumonia (64 isolates; 62.5% community vs. 37.5% hospital) and Pseudomonas aeruginosa (42 isolates; 50% each from community and hospital). The gram-positive bacteria primarily included Streptococcus species (100 isolates; 83(83.0%) community vs. 17(17.0%) hospital) and Enterococcus faecalis (72 isolates; 58(80.6%) community vs. 14(19.4%) hospital). Overall, gram-positive bacteria comprised 149 isolates, with a higher occurrence in community settings 284(75.3%) compared to hospitals 93(24.7%) (Table 2).
|
Gram-Positive/Negative |
Microorganism N (%) |
Source of Infection | Total | |
| Community | Hospital | |||
| Gram Negative |
Escherichia coli |
179(73.1) |
66(26.9) |
245(100.0) |
|
Klebsiella pneumonia |
40(62.5) |
24(37.5) |
64(100.0) |
|
|
Pseudomonas aeruginosa |
21(50.0) |
21(50.0) |
42(100.0) |
|
|
Proteus species |
12(70.6) |
5(29.4) |
17(100.0) |
|
|
Morganella morganii |
7(77.8) |
2(22.2) |
9(100.0) |
|
|
Citrobacter species |
7(87.5) |
1(12.5) |
8(100.0) |
|
|
Achromobacter spp. |
3(37.5) |
5(62.5) |
8(100.0) |
|
|
Moraxella species |
5(62.5) |
3(37.5) |
8(100.0) |
|
|
Klebsiella species |
4(57.1) |
3(42.9) |
7(100.0) |
|
|
Serratia species |
4(66.7) |
2(33.3) |
6(100.0) |
|
|
Salmonella species |
4(80.0) |
1(20.0) |
5(100.0) |
|
|
Enterobacter species |
4(80.0) |
1(20.0) |
5(100.0) |
|
|
Burkholderia cepacia |
1(20.0) |
4(80.0) |
5(100.0) |
|
|
Acinetobacter species |
1(25.0) |
3(75.0) |
4(100.0) |
|
|
Cedecea davisae |
2(100.0) |
0(0.0) |
2(100.0) |
|
|
Pasteurella multocida |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Pseudomonas aeruginosa |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Alloiococcus otitidis |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Arcanobacterium species |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Alcaligenes faecalis |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Pasteurella multocida |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Providencia rettgeri |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Vibrio vulnificus |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Pantoea agglomerans |
2(100.0) |
0(0.0) |
2(100.0) |
|
|
Pseudomonas species |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Rhizobium radiobacter |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Stenotrophomonas maltophilia |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Tatumella ptyseos |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Total |
301(67.0) |
148(33.0) |
449(100.0) |
|
| Gram Positive |
Streptococcus species |
83(83.0) |
17(17.0) |
100(100.0) |
|
Enterococcus faecalis |
58(80.6) |
14(19.4) |
72(100.0) |
|
|
Staphylococcus haemolyticus |
46(79.3) |
12(20.7) |
58(100.0) |
|
|
Staphylococcus epidermidis |
31(64.6) |
17(35.4) |
48(100.0) |
|
|
Staphylococcus aureus |
27(57.4) |
20(42.6) |
47(100.0) |
|
|
Staphylococcus species |
15(71.4) |
6(28.6) |
21(100.0) |
|
|
Corynebacterium species |
9(69.2) |
4(30.8) |
13(100.0) |
|
|
Arcanobacterium species |
4(100.0) |
0(0.0) |
4(100.0) |
|
|
Lactobacillus species |
3(100.0) |
0(0.0) |
3(100.0) |
|
|
Pediococcus pentosaceus |
0(0.0) |
2(100.0) |
2(100.0) |
|
|
Micrococcus lylae |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Alloiococcus otitidis |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Bacillus circulans |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Gemella morbillorum |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Kytococcus sedentarius |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Kocuria Kristinae |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Rothia dentocariosa |
1(100.0) |
0(0.0) |
1(100.0) |
|
|
Sreptococcus species |
0(0.0) |
1(100.0) |
1(100.0) |
|
|
Total |
284(75.3) |
93(24.7) |
377(100.0) |
|
Antibiotic sensitivity and resistance in community setting
In community settings, among the tested gram-positive isolates, the highest sensitivity rates were observed for imipenem 95(96.9%), followed closely by linezolid at 151(95.6%), meropenem at 96 isolates (94.1%), tigecycline at 61 isolates (93.9%), and daptomycin at 59 isolates (93.7%). Conversely, the highest antibiotic resistance rates were recorded for azithromycin 19(90.5%), followed by ofloxacin 19 isolates (76.0%), and cefixime 68 isolates (74.7%). The overall resistance rate among gram-positive isolates was 1732 (38.7%). For gram-negative isolates, sensitivity rates were as follows: meropenem at 260 isolates (94.5%), tigecycline at 61(93.9%), imipenem at 225(85.2%), and amikacin at 128(81.5%). Notably, high resistance rates were seen, with 20 isolates (100.0%) resistant to clindamycin and 119 isolates (92.2%) resistant to ampicillin. The overall resistance rate among gram-negative isolates was 1614 (37.7%) (Suppl 1).
Antibiotic sensitivity and resistance in hospital isolates
In hospital settings, gram-positive isolates exhibited the highest sensitivity to daptomycin (43 isolates, 93.5%), followed by linezolid (62 isolates, 92.5%), teicoplanin (54 isolates, 91.5%), and tigecycline (37 isolates, 90.2%). The most significant resistance rates were observed for azithromycin (11 isolates, 84.6%) and cefixime (16 isolates, 80.0%). The overall antibiotic resistance rate among gram-positive isolates was 727 (41.1%). For gram-negative isolates, the highest sensitivity rates were noted for meropenem (108 isolates, 85.0%), imipenem (103 isolates, 79.8%), amikacin (80 isolates, 73.4%), and piperacillin-tazobactam (88 isolates, 71.5%). However, resistance was notably high for ampicillin (84 isolates, 95.6%) and cefazolin (80 isolates, 85.1%). The overall resistance rate among gram-negative isolates was 1044 (50.8%) (Suppl 2).
MDR rates in community-acquired infections
In the community setting, MDR among gram-negative bacterial isolates was observed in 183 cases (63.1%). Notably, all Morganella morganii isolates (7, 100.0%) and 3(75.0%) of Klebsiella species and Salmonella species were classified as MDR. Among gram-positive isolates, MDR was present in 171 cases (61.9%), with Lactobacillus species showing 100.0% MDR (3 isolates) and Staphylococcus aureus exhibiting a high MDR rate, with 21 out of 27 isolates (77.8%). Overall, the MDR rate in community-acquired infections was 62.5% (Suppl 3).
MDR rates in hospital-acquired infections
In the hospital setting, MDR was observed in 113 gram-negative bacterial isolates (86.2%). Notably, all isolates of Proteus species, Burkholderia cepacia, and Achromobacter species (100%) were classified as MDR. Among gram-positive isolates, 59 cases (68.6%) exhibited MDR, with Staphylococcus haemolyticus showing an MDR rate of 83.3% (10 out of 12 isolates) and Enterococcus faecalis at 78.6% (11 out of 14 isolates). Overall, the MDR rate in hospital-acquired infections was 79.3% (Suppl 3).
Risk factors for community vs. hospital-acquired infections
In the analysis of risk factors for community-acquired versus hospital-acquired infections, males had a significantly higher proportion of hospital-acquired infections, with 688 (75.4%) compared to 374 (61.0%) in community-acquired infections (p<0.001). Individuals over 40 years old were more likely to have hospital-acquired infections, 280 (35.2%) versus 183(25.1%) in the community-acquired group (p<0.001). Patients with pre-existing comorbidities, including diabetes, malignancy, obesity, hypertension, renal insufficiency, heart failure, and asthma, demonstrated significantly higher rates of hospital-acquired infections (p < 0.05) (Table 3).
| Risk Factors | Infection Source | P-Value | |
|
Community acquired |
Hospital acquired |
||
|
Gender (N, %) Male Female |
688(75.4) |
224(24.6) |
<0.001 |
|
Age <40 |
516(64.8) |
280(35.2) |
<0.001 |
|
Diabetes Yes No |
903(73.2) |
331(26.8) |
<0.001 |
|
Malignancy Yes No |
1005(71.8) |
395(28.2) |
|
|
Obesity Yes No |
890(71.0) |
364(29.0) |
0.034 |
|
Hypertension Yes No |
877(73.5) |
316(26.5) |
<0.001 |
|
Renal Insufficiency Yes No |
961(71.9) |
375(28.1) |
<0.001 |
|
Heart Failure Yes |
966(72.6) |
364(27.4) |
<0.001 |
|
Pregnancy Yes No |
1010(68.7) |
460(31.3) |
<0.001 |
|
Asthma Yes No |
1040(70.4) |
437(29.6) |
0.001 |
Discussion
Antimicrobial resistance (AMR) has become one of the most critical global public health challenges of the 21st century. It arises when microorganisms resist antimicrobial drugs such as antibiotics, rendering these treatments ineffective. This resistance primarily results from the overuse and misuse of antibiotics in various sectors, including clinical settings. Often referred to as the "Silent Pandemic," AMR demands immediate and effective action rather than being treated as a distant concern [13]. Despite the growing threat of antimicrobial resistance, the overuse of these agents remains prevalent, particularly in patients with critical illnesses, advanced disease stages, malignancies, or immunocompromised conditions [14].
Hospitals are recognized as high-risk environments for health, particularly due to the prevalence of HAIs in both developed and developing countries [15]. The impact of HAIs is substantial, contributing to increased healthcare costs, greater disease severity, higher rates of antimicrobial resistance, and elevated morbidity and mortality. Within healthcare settings, bacterial pathogens are the primary culprits behind nosocomial infections, with many strains exhibiting resistance to both standard and last-resort antibiotics [16].
Gram-negative bacteria are frequently involved in HAIs, accounting for up to 87% of cases [15]. Among Gram-positive bacteria, Staphylococcus aureus is the most prevalent strain [17]. In Europe and Asia, the most common Gram-negative pathogens include Pseudomonas aeruginosa, Acinetobacter baumannii, and members of the Enterobacteriaceae family [18,19]. A multicenter retrospective study conducted across five private hospitals in Lebanon, involving 258 patients, reported that Escherichia coli and Pseudomonas aeruginosa were the most prevalent Gram-negative bacteria, while Staphylococcus aureus was the dominant Gram-positive isolate [1]. Similarly, the present study found that Gram-negative bacteria accounted for 62.1% (148 out of 241) of hospital-acquired infections (HAIs). The most frequently isolated Gram-negative pathogens were Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Among Gram-positive bacteria, Staphylococcus aureus emerged as the most prevalent strain in the hospital setting.
Hospital-acquired microorganisms exhibited greater resistance to antimicrobials than their community-acquired counterparts. For instance, a study by Matta et al. demonstrated that hospital-acquired Pseudomonas aeruginosa showed significantly higher resistance to all evaluated antimicrobial agents thanacquired strains [1]. In the current study, the resistance rate among community-acquired infections was 38.2% (3,346 out of 8,760 isolates), whereas the resistance rate among hospital-acquired infections was 46.3% (1,771 out of 3,825 isolates).
Escherichia coli infections are typically treated with antibiotics such as ciprofloxacin, levofloxacin, and other fluoroquinolones; however, resistance to multiple antibiotics has become increasingly prevalent. The growing resistance to fluoroquinolones and the emergence of extended-spectrum beta-lactamases pose significant challenges in managing these infections. Although carbapenems are generally considered the preferred treatment for MDR Escherichia coli infections, reports of resistance to carbapenems are also rising [15]. In this study, sensitivity rates for Escherichia coli isolates in community-acquired infections were found to be 49.4%, 53%, and 60.3% for ciprofloxacin, levofloxacin, and norfloxacin, respectively. In contrast, sensitivity rates among hospital-acquired isolates were lower, with 29.7%, 33.3%, and 28.6% for the same antibiotics. Furthermore, sensitivity to imipenem and meropenem was observed in 91.9% and 96.0% of community-acquired Escherichia coli isolates, while sensitivity in hospital-acquired cases was notably lower at 83.3% and 87.9%. These findings indicate a concerning trend of increased antibiotic resistance among Escherichia coli isolates from hospital settings, particularly concerning carbapenem resistance.
Klebsiella pneumoniae is the second most prevalent cause of HAIs, following Escherichia coli [15]. While it is primarily considered an opportunistic pathogen, there has been a notable increase in its hypervirulence, often linked to hypercapsulation [20], along with a rise in antibiotic resistance [21]. The emergence of carbapenem-resistant Klebsiella pneumoniae strains poses a significant global health threat, contributing to increased mortality rates primarily due to the acquisition of Klebsiella pneumoniae carbapenemases [22]. Multidrug-resistant strains can exhibit resistance to all beta-lactams and fluoroquinolones. Consequently, last-resort treatment options often involve polymyxin B, frequently in combination with tigecycline or certain aminoglycosides [15]. In this study, community-acquired Klebsiella pneumoniae isolates showed a sensitivity rate of 100% to tigecycline, whereas the sensitivity among hospital-acquired isolates was significantly lower at 68.4%. Additionally, fewer than 50% of K. pneumoniae isolates demonstrated sensitivity to all beta-lactam antibiotics.
A study conducted in India investigating the etiology and antimicrobial sensitivity of organisms responsible for community-acquired pneumonia, which included 145 patients, found Streptococcus infections to be the most frequently isolated pathogen in the community setting [23]. In line with these findings, the current study also identified Streptococcus infections as one of the most commonly isolated pathogens within the community context. This could be explained by high transmissibility, opportunistic nature in vulnerable populations, association with diverse infections, seasonal peaks, and the dynamics of antimicrobial resistance and vaccination.
In recent decades, the prevalence of antimicrobial resistance has escalated worldwide, with MDR bacteria emerging as a significant cause of nosocomial infections. The risk of MDR infections is linked to several factors, including prolonged antimicrobial therapy, cross-transmission, extended hospital stays, and invasive procedures. These resistant bacteria can lead to various infections—such as pneumonia, urinary tract infections, and wound infections—associated with increased morbidity, and mortality [24]. In this study, a higher MDR was found among HAIs compared to Community acquired infections, with 79.3% for HAI and 62.5% for Community acquired setting. Higher rates of MDR in HAIs compared to community-acquired infections result from factors such as prolonged antibiotic use, invasive procedures, and close patient proximity, which foster the emergence and spread of resistant strains. A retrospective study conducted in a tertiary general hospital in Jining, China, revealed a high prevalence of MDR HAIs; out of 7,579 bacterial isolates, 3,223 (42.5%) were identified as MDR. Gram-negative bacteria were the most frequently isolated MDR pathogens, with Escherichia coli exhibiting the highest detection rate at 37.7%. Collectively, Escherichia coli and Klebsiella pneumoniae accounted for 51.0% of all MDR isolates [24]. In this study, the prevalence of MDR among hospital settings was found to be 79.3%, with 172 out of 217 isolates classified as MDR. Consistent with previous findings, gram-negative bacteria were the most frequently isolated MDR pathogens, with Escherichia coli detected in 24.9% (54 out of 217) of cases, followed by Klebsiella pneumoniae at 10.1% (22 out of 217).
Multidrug-resistant Staphylococcus aureus is a leading cause of HAIs and a significant contributor to mortality among hospitalized patients, largely due to its possession of resistance genes against various antibiotics, including commonly used anti-staphylococcal drugs [25]. In this study, 25.4% (15 out of 59) of the MDR Gram-positive isolates from hospital settings were identified as multidrug-resistant Staphylococcus aureus. Most Staphylococcus aureus isolates exhibited resistance to penicillin, while all were sensitive to the carbapenems.
A prospective cohort study conducted over one year at a university tertiary care hospital in Portugal identified neoplastic diseases, including hematologic malignancies and solid tumors, as well as immunocompromised states, as common conditions associated with hospital-acquired infections [26]. Notably, no gender differences were observed in infection rates [1]. In this study, patients with pre-existing comorbidities such as diabetes, malignancy, obesity, hypertension, renal insufficiency, heart failure, and asthma were found to have significantly higher rates of hospital-acquired infections.
Conclusion
Multidrug-resistant infections were prevalent in HAIs, with most isolates resistant to current antibiotics. This underscores the need for enhanced surveillance to optimize antibiotic use and control HAIs. The higher resistance in HAIs compared to community-acquired infections highlights the importance of early detection of resistance.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The study's ethical approval was obtained from the scientific committee of the Kscien Organization for Scientific Research.
Patient consent (participation and publication): Verbal informed consent was obtained from patients for participation in this study and publication.
Source of Funding: Saaeda company.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: BAA and FHK were significant contributors to the conception of the study and the literature search for related studies. RQS, HAY, WAH and SHK were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. AMM, FA, and KKM were involved in the literature review, study design, and manuscript writing. DQM, BHI, HSA, SHA, MOS and SSA Literature review, final approval of the manuscript, and processing of the tables. RQS and AMM confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.
References
- Matta R, Hallit S, Hallit R, Bawab W, Rogues AM, Salameh P. Epidemiology and microbiological profile comparison between community and hospital acquired infections: a multicenter retrospective study in Lebanon. Journal of infection and public health. 2018;11(3):405-11.doi:10.1016/j.jiph.2017.09.005
- Chen Y, Xu X, Liang J, Lin H. Relationship between climate conditions and nosocomial infection rates. African Health Sciences. 2013;13(2):339-43. doi:10.4314/ahs.v13i2.20
- Alp E, Leblebicioglu H, Doganay M, Voss A. Infection control practice in countries with limited resources. Annals of clinical microbiology and antimicrobials. 2011; 10:1-4. doi:10.1186/1476-0711-10-36
- Cornejo-Juárez P, Vilar-Compte D, Pérez-Jiménez C, Ñamendys-Silva SA, Sandoval-Hernández S, Volkow-Fernández P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. International Journal of Infectious Diseases. 2015; 31:31-4. doi:10.1016/j.ijid.2014.12.022
- Rosenthal VD, Belkebir S, Zand F, Afeef M, Tanzi VL, Al-Abdely HM, et al. Six-year multicenter study on short-term peripheral venous catheters-related bloodstream infection rates in 246 intensive units of 83 hospitals in 52 cities of 14 countries of Middle East: Bahrain, Egypt, Iran, Jordan, Kingdom of Saudi Arabia, Kuwait, Lebanon, Morocco, Pakistan, Palestine, Sudan, Tunisia, Turkey, and United Arab Emirates—International Nosocomial Infection Control Consortium (INICC) findings. Journal of infection and public health. 2020;13(8):1134-41. doi:10.1016/j.jiph.2020.03.012
- Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, Abdollahi B, Seyghalani Talab F, Sanaei M, Zarabi F, Dolati Y. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS One. 2023;18(1):e0274248. doi:10.1371/journal.pone.0274248
- Gugliotta C, Deiana G, Dettori M, Sotgiu G, Azara A, Castiglia P. Prevalence study on health-care associated infections and on the use of antimicrobials carried out with the light protocol of the European Centre for Disease Prevention and Control. Ann Ig. 2020;32(4):357-67. doi:10.7416/ai.2020.2359
- Berglund Kristiansson E, Källman U. Healthcare staff's views on the patients' prerequisites to be co‐creator in preventing healthcare‐associated infections. Scandinavian journal of caring sciences. 2020;34(2):314-21. doi:10.1111/scs.12730
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. American journal of infection control. 2008;36(5):309-32. doi:10.1016/j.ajic.2008.03.002
- Hong JS, Kim D, Kang DY, Park BY, Yang S, Yoon EJ, et al. Evaluation of the BD Phoenix M50 automated microbiology system for antimicrobial susceptibility testing with clinical isolates in Korea. Microbial Drug Resistance. 2019;25(8):1142-8. doi:10.1089/mdr.2018.0370
- Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. Journal of clinical microbiology. 2020;58(3):10-128. doi:10.1128/jcm.01864-19
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection. 2012;18(3): 268-81. doi:10.1111/j.1469-0691.2011.03570.x
- Ahmed SK, Hussein S, Qurbani K, Ibrahim RH, Fareeq A, Mahmood KA, et al. Antimicrobial resistance: impacts, challenges, and future prospects. Journal of Medicine, Surgery, and Public Health. 2024; 2:100081. doi:10.1016/j.glmedi.2024.100081
- Majumder MA, Rahman S, Cohall D, Bharatha A, Singh K, Haque M, et al. Antimicrobial stewardship: fighting antimicrobial resistance and protecting global public health. Infection and drug resistance. 2020:4713-38. doi:10.2147/IDR.S290835
- Abban MK, Ayerakwa EA, Mosi L, Isawumi A. The burden of hospital acquired infections and antimicrobial resistance. Heliyon. 2023. doi:10.1016/j.heliyon.2023.e20561
- Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. New England Journal of Medicine. 2010;362(19):1804-13. doi:10.1056/NEJMra0904124
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. Jama. 2009;302(21):2323-9. doi:10.1001/jama.2009.1754
- World Health Organization. Antimicrobial resistance: global report on surveillance. World Health Organization; 2014. https://iris.who.int/bitstream/handle/10665/112642/?sequence=1
- Saleem Z, Godman B, Hassali MA, Hashmi FK, Azhar F, Rehman IU. Point prevalence surveys of health-care-associated infections: a systematic review. Pathogens and global health. 2019;113(4):191-205. doi:10.1080/20477724.2019.1632070
- Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107-18. doi:10.4161/viru.22718
- Flores-Valdez M, Ares MA, Rosales-Reyes R, Torres J, Girón JA, Weimer BC, et al. Whole genome sequencing of pediatric Klebsiella pneumoniae strains reveals important insights into their virulence-associated traits. Frontiers in Microbiology. 2021; 12:711577. doi:10.3389/fmicb.2021.711577
- Su S, Zhang J, Zhao Y, Yu L, Wang Y, Wang Y, et al. Outbreak of KPC-2 Carbapenem-resistant Klebsiella pneumoniae ST76 and Carbapenem-resistant K2 Hypervirulent Klebsiella pneumoniae ST375 strains in Northeast China: molecular and virulent characteristics. BMC Infectious Diseases. 2020; 20:1-4. doi:10.1186/s12879-020-05143-y
- Menon RU, George AP, Menon UK. Etiology and anti-microbial sensitivity of organisms causing community acquired pneumonia: a single hospital study. Journal of family medicine and primary care. 2013;2(3):244-9. doi:10.4103/2249-4863.120728
- Wang M, Wei H, Zhao Y, Shang L, Di L, Lyu C, et al. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosnian journal of basic medical sciences. 2019;19(1):86. doi:10.17305/bjbms.2018.3826
- Otto M. Community-associated MRSA: what makes them special?. International Journal of Medical Microbiology. 2013;303(6-7):324-30. doi:10.1016/j.ijmm.2013.02.007
- Cardoso T, Ribeiro O, Aragão I, Costa-Pereira A, Sarmento A. Differences in microbiological profile between community-acquired, healthcare-associated and hospital-acquired infections. Acta Médica Portuguesa. 2013; 26:377-84. https://www.actamedicaportuguesa.com/revista/index.php/amp/article/view/208/3711doi:10.3892/br.2024.1805.

This work is licensed under a Creative Commons Attribution 4.0 International License.

