Clinicopathological Features of Indeterminate Thyroid Nodules: A Single-center Cross-sectional Study
Abstract
Introduction
Due to indeterminate cytology, Bethesda III is the most controversial category within the Bethesda System for Reporting Thyroid Cytopathology. This study examines the characteristics and malignancy rates of thyroid nodules (TNs) classified as Bethesda III.
Methods
Data were collected by reviewing electronic medical records, encompassing demographic details, medical history, chief complaint, laboratory tests (including thyroid function tests), preoperative imaging, cytology results, management, and histopathology diagnosis.
Results
The majority of the cases were female (84.7%). Patients’ ages ranged from 15 to 71 years, with a mean of 42.9 ± 10.5 years. Regarding goiter grading, 37 cases (21.8%) were classified as G0, 62 (36.5%) as G1, 55 (32.3%) as G2, and seven (4.1%) as G3. Thyroid Imaging Reporting and Data Systems scoring categorized the nodules as TI-RADS 2 (5.3%), TI-RADS 3 (40%), TI-RADS 4 (38.2%) and TI-RADS 5 (9.4%). The size of TNs on ultrasound ranged from 0.3 cm to 7.8 cm, with a mean size of 2.06 ± 1.3 cm. Adenoma was the most common diagnosis (40%), followed by thyroiditis (16.5%), papillary thyroid carcinoma (15.9%), and papillary thyroid microcarcinoma (15.9%). The nodules were predominantly benign (64.7%), while 35.3% were malignant. Patients with malignant nodules were younger than those with benign nodules (p=0.044). Benign nodules were significantly larger than malignant ones (p-value = 0.003).
Conclusion
One of three TNs with indeterminate cytology may be malignant. Patients with malignant nodules tend to be younger than those with benign nodules, and benign nodules are likely larger than malignant ones.
Introduction
The global incidence of thyroid nodules (TNs) is estimated to range between 20% and 60%, varying by gender, age, and geographic location [1]. Almost 90–95% of these nodules are benign and asymptomatic at diagnosis and remain so during follow-up [2]. However, the incidence of thyroid cancer, including papillary thyroid carcinoma (PTC) and papillary thyroid microcarcinoma (PTMC), has risen concurrently with the advancements in diagnostic technology and enhanced surveillance. Additionally, incidence-based mortality from thyroid cancer has increased, with an annual percent change of 1% [1,3]. With new ultrasound (U/S) technology and the widespread use of high-resolution scanners, detecting TNs has become much easier. However, for many sonographers, the primary challenge lies in accurately distinguishing malignant TNs from benign ones. To address this, certain U/S characteristics, such as unclear borders, micro-calcifications, irregular shapes, solid components, and internal echoes, are commonly used to assess the malignancy risk of nodules. Nonetheless, relying on any single characteristic alone is insufficient to accurately differentiate between malignant and benign nodules [4]. Fine needle aspiration cytology (FNAC) has become the standard modality for assessing thyroid nodular pathology [1]. In 2008, the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) was introduced to standardize the cytological evaluation of TNs. The BSRTC categorizes diagnoses into six classes with progressively higher suspicion for malignancy: nondiagnostic (Class I), benign (Class II), atypia of undetermined significance /follicular lesion of undetermined significance (AUS/FLUS) (Class III), follicular neoplasm/oncocytic cell neoplasm (Class IV), suspicious for malignancy (Class V), and malignant (Class VI) [5]. The most controversial category within the BSRTC is AUS/FLUS due to indeterminate cytology. Despite the routine use of cytological examination in evaluating TNs, which has reduced the overall number of patients needing diagnostic surgery, a significant percentage still undergo surgery to obtain a definitive histological diagnosis [1]. The reported malignancy rates for AUS and FLUS exhibit considerable variability [3]. This study examines the characteristics and malignancy rates of TNs classified as Bethesda III. The referenced studies have been checked to avoid citing non-peer-reviewed data [6].
Methods
Study design
This retrospective, single-center, cross-sectional study was conducted at Smart Health Tower between August 2024 and September 2024. The patients gave verbal informed consent to publish their data in this study. The ethical board at Kscien organization approved the study with approval number 26 on August 2024.
Data collection
Data were collected by reviewing electronic medical records, encompassing demographic details, medical history, chief complaints, laboratory tests (including thyroid function tests), preoperative imaging, FNAC results, management, and final histological diagnosis.
Eligibility criteria
The study included patients with TNs classified as Bethesda III on FNAC who subsequently underwent surgery for a definitive histopathological diagnosis. Patients with incomplete medical documentation, including clinical, radiological, and FNAC data, as well as those with recurrent or a history of thyroid cancer, were excluded.
Statistical Analysis
The data were collated in a Microsoft Excel (2021) sheet and then transferred into version 27 of Statistical Package for Social Sciences (SPSS). The chi-squared and Fisher's exact tests were used to analyze categorical data with independent samples t-test for quantitative variables. The data were presented as frequency, percentage, mean with standard deviation, and median with ranges. The level of significance was set at p-value <0.05.
Results
Patient demography and presentation
The study included 170 patients, of whom the majority were female (84.7%). Their ages ranged from 15 to 71 years, with a mean of 42.9 ± 10.5. In total, 157 patients (92.3%) were married, 11 (6.5%) were unmarried, and two individuals (1.2%) were either divorced or widowed. Most of the cases (65.9%) were housewives. Seven cases were smokers (4.1%), while 20 were passive smokers (11.8%), and one (0.6%) was an ex-smoker. The reason for the presentation was having a thyroid disease and visiting the hospital for follow-up in most of the cases (38.2%), followed by neck swelling (34.7%) and fatigue (10.0%) (Table 1). Regarding goiter grading, 37 cases (21.8%) were classified as G0, 62 (36.5%) as G1, 55 (32.3%) as G2, and seven (4.1%) as G3. Nine patients (5.3%) had no goiter grading available (Table 2).
|
Variables |
Frequency / Percentage |
|
Patient demographics |
|
|
Age range (median, mean ± SD), years |
15 – 71 (43, 42.9 ± 10.5) |
|
Sex Male Female |
26 (15.3%) 144 (84.7%) |
|
Marital status Unmarried Married Divorced/Widow |
11 (6.5%) 157 (92.3 %) 2 (1.2%) |
|
Occupation Housewife Teacher Worker Unemployed Policeman Student Doctor Retired Others |
112 (65.9%) 16 (9.4%) 15 (8.8%) 7 (4.1%) 4 (2.3 %) 3 (1.8%) 2 (1.2%) 2 (1.2%) 9 (5.3%) |
|
Smoking status Smoker Passive smoker Ex-smoker Non-smoker |
7 (4.1%) 20 (11.8%) 1 (0.6%) 142 (83.5%) |
|
Chief complaint Follow-up Neck swelling Fatigue Neck pain Palpitation Dysphagia Exophthalmos Hair loss Sweating Weigh gain N/A |
65 (38.2%) 59 (34.7%) 17 (10.0%) 10 (5.9 %) 4 (2.3 %) 3 (1.8 %) 3 (1.8 %) 2 (1.2 %) 1 (0.6 %) 1 (0.6 %) 5 (2.9%) |
|
Variables |
Frequency / Percentage |
|
Goiter grading G0 G1 G2 G3 N/A |
37 (21.8 %) 62 (36.5 %) 55 (32.3 %) 7 (4.1%) 9 (5.3 %) |
|
Thyroid state Euthyroid Hypothyroidism Hyperthyroidism N/A |
87 (51.2 %) 42 (24.7%) 29 (17.1%) 12 (7.0%) |
|
Feature on ultrasound Solid Cystic Mixed N/A |
113 (66.5 %) 1 (0.6 %) 32 (18.8%) 24 (14.1%) |
|
TI-RADS score TR2 TR3 TR4 TR5 N/A |
9 (5.3%) 68 (40.0%) 65 (38.2%) 16 (9.4%) 12 (7.1%) |
|
Tumor size on ultrasound (range, mean ± SD), cm |
0.3 - 7.8, 2.06 ± 1.3 |
|
Tumor size group <1 cm 1-2 cm >2-3 cm >3-4 cm >4 cm N/A |
35 (20.6%) 61 (35.9%) 34 (20.0%) 22 (12.9 %) 15 (8.8%) 3 (1.8 %) |
|
Fine needle aspiration Bethesda III |
170 (100.0%) |
|
Management Total thyroidectomy Lobectomy Nodulectomy Isthmusectomy |
115 (67.6 %) 30 (17.6 %) 21 (12.4 %) 4 (2.4 %) |
|
Diagnosis Adenoma Thyroiditis PTC PTMC MNG NIFTP FTC Graves’ disease Collision tumor (PTMC and follicular adenoma) MTC |
68 (40.0%) 28 (16.5 %) 27 (15.9 %) 27 (15.9 %) 7 (4.1%) 5 (2.9%) 4 (2.3%) 2 (1.2 %) 1 (0.6 %) 1 (0.6 %) |
|
Nature of tumor Benign Malignant |
110 (64.7 %) 60 (35.3 %) |
|
N/A: Non-available, PTMC: Papillary thyroid microcarcinoma, PTC: Papillary thyroid carcinoma, FTC: Follicular thyroid carcinoma, MTC: Medullary thyroid carcinoma, MNG: multinodular goiter, NIFTP: Noninvasive follicular thyroid neoplasm with papillary-like nuclear features. |
|
Diagnosis and management
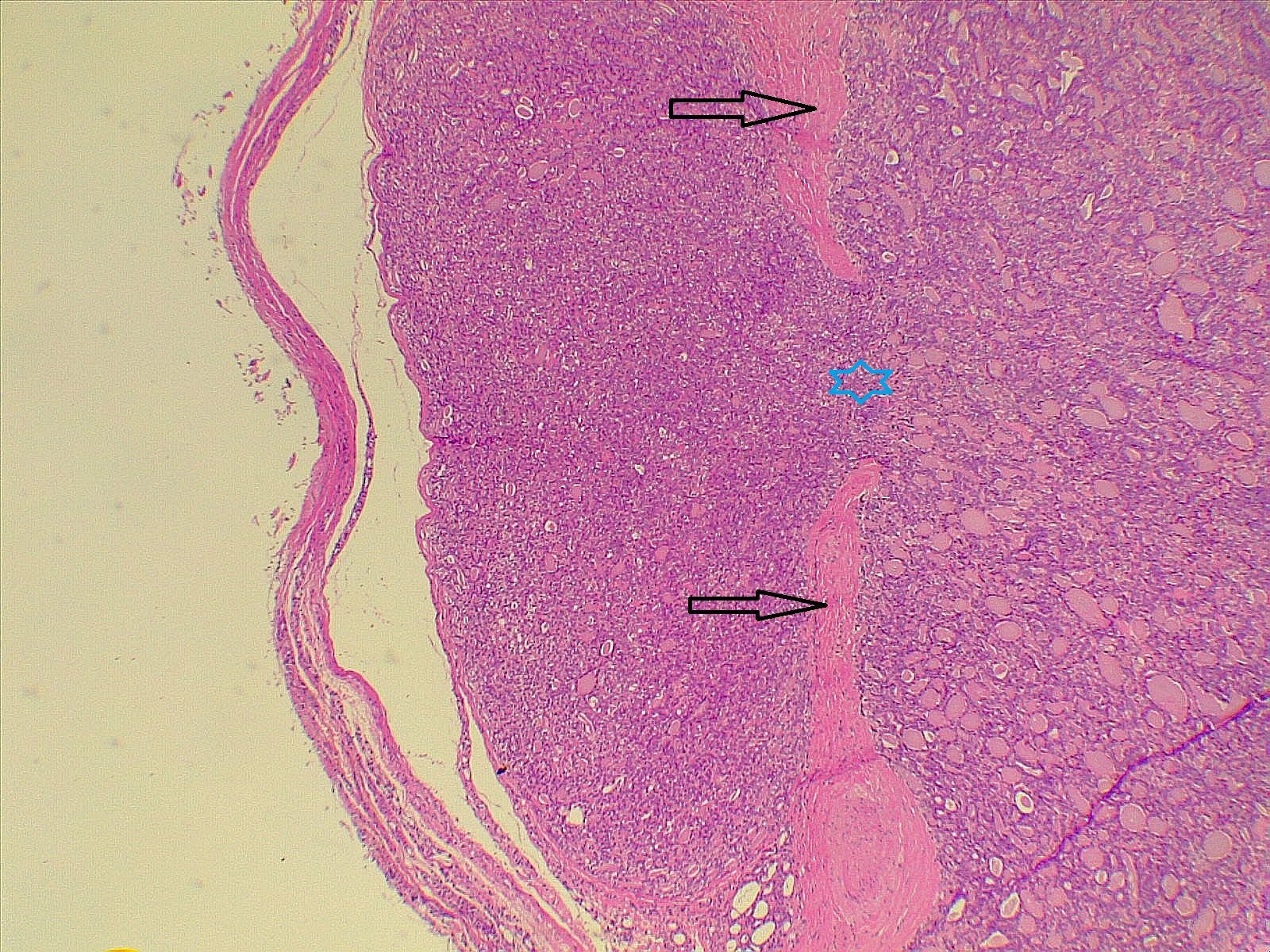
Thyroid function assessment revealed that the majority of the participants were euthyroid (51.2%). However, hypothyroidism and hyperthyroidism were observed in 24.7% and 17.1% of patients, respectively. Ultrasonography of the thyroid showed that 113 patients (66.5%) had solid nodules, while 32 (18.8%) presented with mixed (solid and cystic) nodules. Only one case (0.6%) had a purely cystic nodule. The content of the tumor in the remaining cases was unknown (14.1%). Thyroid Imaging Reporting and Data Systems (TI-RADS) scoring categorized the nodules as TI-RADS 2 (5.3%), TI-RADS 3 (40%), TI-RADS 4 (38.2%) and TI-RADS 5 (9.4%). It was unknown in 12 cases (7.1%). The size of the TNs on U/S ranged from 0.3 cm to 7.8 cm, with a mean of 2.06 ± 1.3 cm. The majority of the cases were managed by total thyroidectomy (67.6%), followed by lobectomy (17.6%), nodulectomy (12.4%), and isthmusectomy (2.4%). Adenoma (Figure 1) was the most common diagnosis, identified in 68 cases (40%), followed by thyroiditis (16.5%), PTC (15.9%) (Figure 2), and PTMC (15.9%) (Figure 3). Benign multinodular goiter (MNG) was diagnosed in seven individuals (4.1%), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in five patients (2.9%) (Figure 4), and follicular thyroid carcinoma (Figure 5) in four cases (2.3%). Other less common diagnoses included Graves’ disease in two patients (1.2%), a collision tumor consisting of PTMC and follicular adenoma in one individual (0.6%), and medullary thyroid carcinoma in another patient (0.6%). The nodules were predominantly benign (64.7%), while 35.3% were malignant (Table 2).
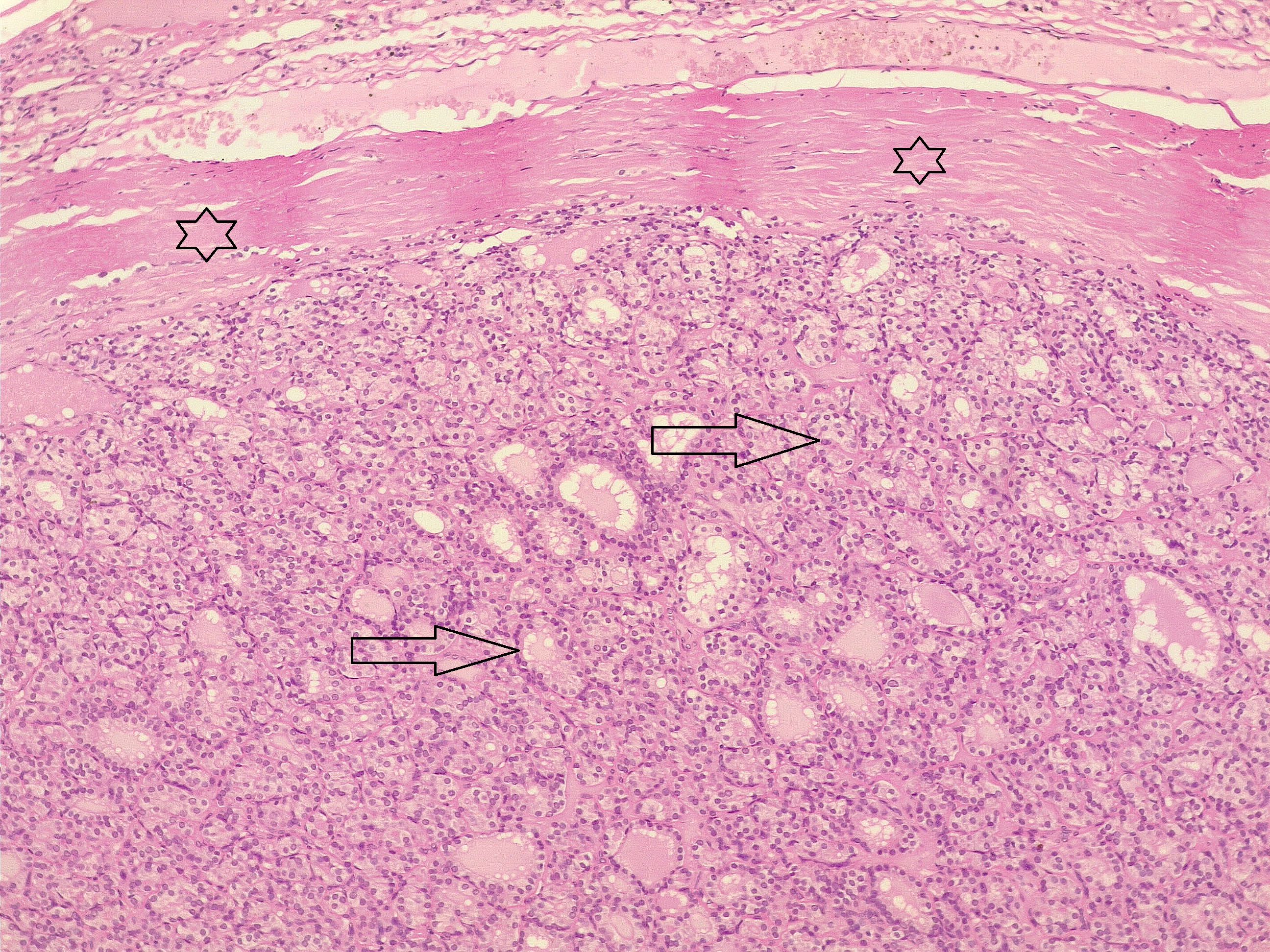
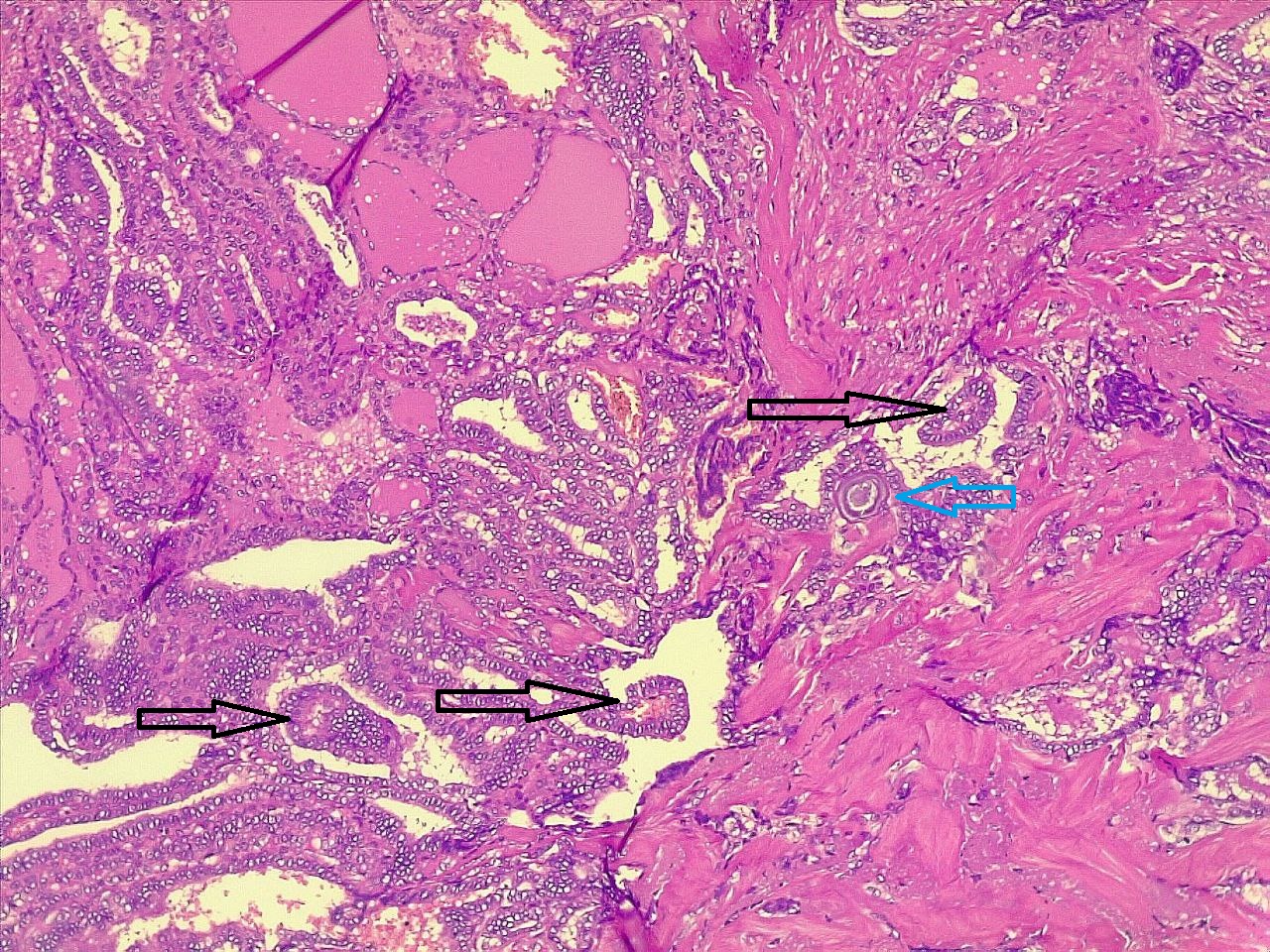
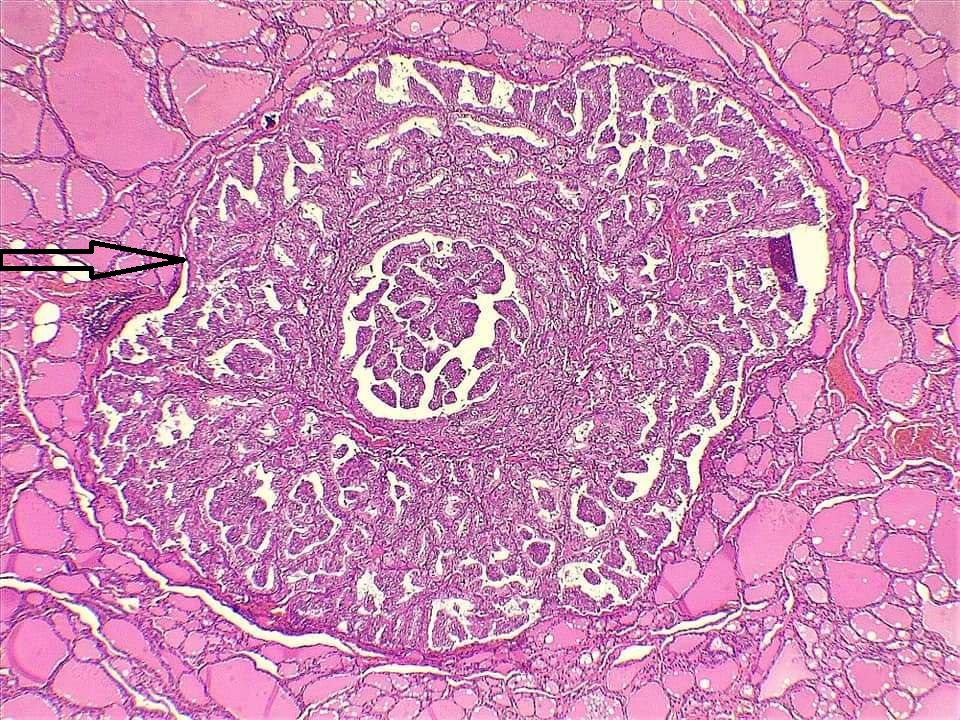
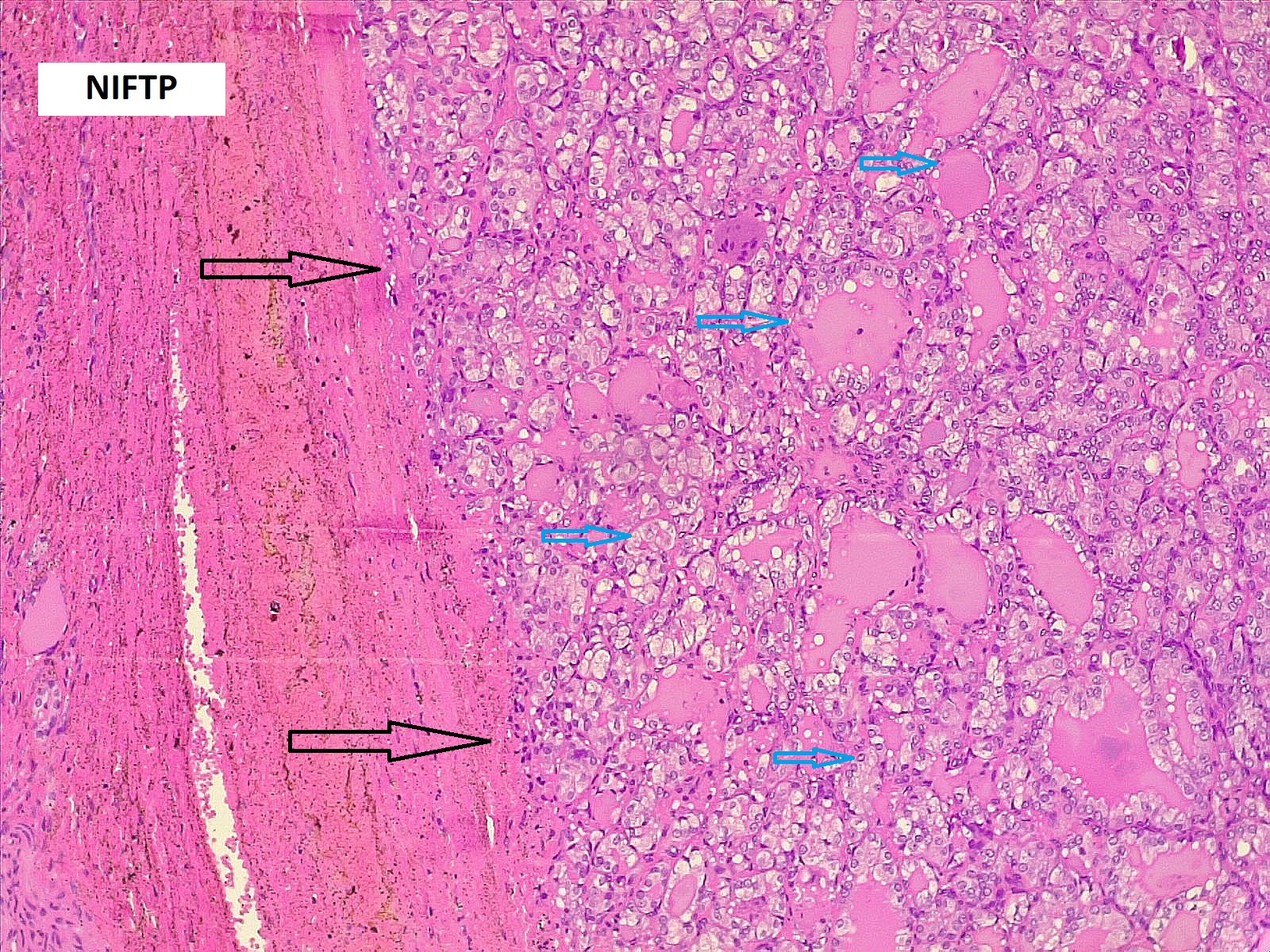
Comparison of patient and tumor characteristics between benign and malignant TNs
In comparing patients' demography and clinical, and radiological characteristics between benign and malignant nodules, no significant differences were observed between the two groups concerning age group, gender, goiter grading, MNG (data not shown), thyroid function, or tumor features on U/S (p-value > 0.05). However, in general, patients with malignant nodules tended to be younger than those with benign nodules (p=0.044). TI-RADS classification differed significantly between benign and malignant nodules. Malignant nodules were significantly associated with TI-RADS categories 4 and 5, whereas benign nodules were predominantly associated with TI-RADS categories 2 and 3 (p-value < 0.001). There was no statistically significant difference in tumor size between benign and malignant nodules when categorized into groups (p-value = 0.053) (Table 3). However, when tumor size was not grouped, benign nodules were significantly larger than malignant ones (p-value = 0.003) (Table 4).
| Variables | Nature of tumor |
Total |
P-value* |
|
|
Benign |
Malignant |
|||
|
Age group 15-25 26-35 36-45 46-55 56-65 >65 |
5 14 42 35 12 2 |
5 12 27 11 4 1 |
10 26 69 46 16 3 |
0.29 |
|
Sex Male Female |
15 95 |
11 49 |
26 144 |
0.50# |
|
Goiter grading G0 G1 G2 G3 |
24 39 37 6 |
13 23 18 1 |
37 62 55 7 |
0.67 |
|
Thyroid state Euthyroid Hyperthyroidism Hypothyroidism |
51 21 31 |
36 8 11 |
87 29 42 |
0.15 |
|
Tumor feature on ultrasound Solid Cystic Mixed |
72 1 24 |
41 0 8 |
113 1 32 |
0.38 |
|
TI-RADS score TR2 TR3 TR4 TR5 |
3 50 43 2 |
1 16 23 14 |
4 66 66 16 |
<0.001 |
|
Tumor size group <1 cm 1-2 cm >2-3 cm >3-4 cm >4 |
17 40 23 15 14 |
18 21 11 7 1 |
35 61 34 22 15 |
0.053 |
|
*Chi-squared test, # Fisher's exact test |
||||
|
Variables |
Number |
Mean |
Std. Deviation |
Std. Error Mean |
P-value** |
|
Tumor size Benign Malignant |
110 58 |
2.28 1.64 |
1.41 1.01 |
0.14 0.13 |
0.003 |
|
Age Benign Malignant |
110 60 |
44.16 40.76 |
10.54 10.22 |
1.0 1.32 |
0.044 |
|
**Independent sample t-test |
|||||
Discussion
Although FNAC is widely utilized, its diagnostic accuracy ranges from 80% to 99%. The ambiguity associated with indeterminate cytological outcomes often leads to uncertainty, and repeating FNAC following a non-diagnostic result remains controversial [7]. Thyroid pathology, especially TNs, primarily affects women. The majority of thyroid cancer patients are women, with an average age of 50 ± 15 years. However, men are more likely to develop aggressive forms of the disease, which are linked to a poorer prognosis [8]. The global incidence of thyroid cancer is on the rise. In 2020, the estimated incidence rate was 10.1 per 100,000 people for women and 3.1 for men, up from 6.1 and 1.9, respectively, in 2012 [8]. Regarding the correlation of gender and age with the risk of malignancy in TNs with indeterminate cytology, controversial findings have been reported [1,3,8,9]. Bessey et al. reported female gender as a risk factor for malignancy [9]; however, Cozzani et al. found that female gender was associated with well-differentiated thyroid cancer predominantly in younger individuals, but this difference diminished in patients over the age of 55 [1]. In contrast, Rano et al. found no effect of gender, race, ethnicity, and underlying thyroid disease on thyroid cancer [8]. Several studies have identified a minimal inverse correlation between patient age and malignancy, suggesting that younger patients with indeterminate nodules face a slightly higher risk of being diagnosed with thyroid cancer [1,8-10]. On the contrary, Dimitriadis et al. reported a similar mean age of 50 between both groups of patients with benign and malignant nodules [3]. In line with existing literature, most of the cases in the present study were female (84.7%); however, gender did not differ significantly between those with malignant and benign nodules. Additionally, no significant difference between malignant and benign nodules was observed when age was categorized into groups. Nonetheless, patients with malignant nodules tended to be younger, with a mean age of 40.76 years, compared to those with benign nodules, who had a mean age of 44.16 years. This finding supports the assumption that TNs in younger patients are more likely to be malignant.
Some scholars have identified iodine deficiency as an indirect risk factor for thyroid cancer. Iodine plays a crucial role in the synthesis of thyroid hormones, and its deficiency can lead to an increase in thyroid volume (goiter) and elevated thyroid-stimulating hormone production. Rano et al. reported a high incidence of goiter among their cases (63%), with malignant histology more commonly associated with MNG (71%) than a single nodule. They found no difference between benign and malignant tumors regarding nodule content (solid, cystic, mixed) on U/S [8]. In the present study, only seven cases (4.1%) had MNG, and contrary to the previous study, there was no association between MNG and an increased risk of malignancy. However, consistent with the findings of Rano et al. [8], nodule content did not differ based on tumor nature, whether benign or malignant.
The usefulness of nodule size as an independent predictor of malignancy remains controversial. Both the British Thyroid Association and the American Thyroid Association recommend total thyroidectomy for indeterminate lesions measuring ≥40 mm due to the associated increased risk of malignancy [11,12]. In a study by Dimitriadis et al., the average nodule size was comparable between benign and malignant subgroups, measuring under 4 cm (3.5 cm vs. 3 cm, respectively). The study also found that the likelihood of a nodule being malignant was similar regardless of its size, whether it was <4 cm or ≥4 cm (27% and 27.7%, respectively) [3]. In line with these findings, some other studies also identified no correlation between nodule size and the risk of malignancy [1,13,14]. Despite that, some others reported different findings. A study from Oxford found that approximately 37% of TNs classified as Bethesda III and measuring over 4 cm were malignant, which was significantly higher than the malignancy rate observed in nodules smaller than 4 cm [15]. Conversely, Cavallo et al. found that larger nodules had a lower malignancy rate and suggested that nodule size should not be considered an independent risk factor for malignancy [16].
Cozzani et al. reported that only 11.6% of the nodules in their study were larger than 4 cm. They attributed this finding to the extensive use of U/S examinations, including those conducted for screening purposes, in their area, which has a very high incidence of thyroid nodular pathology [1]. In the current study, only 8.8% of the cases had nodules greater than 4 cm, which may be due to the reason mentioned by Cozzani et al [1]. Our findings were consistent with the presumption that tumor size in benign nodules may be greater than that of malignant ones.
The literature demonstrates considerable variability in the reported malignancy rates for AUS/FLUS TNs. In a study by Dimitriadis et al., the rate was about 34%, and in the 2017 National British Association of Endocrine and Thyroid Surgeons Audit report, it was 25.7% [3,17]. The Oxford group found that approximately one in four patients with AUS/FLUS cytology was diagnosed with thyroid cancer [15]. Similar results were observed in a systematic review that included 13 studies, revealing a malignancy rate of 22% for cases with Bethesda III cytology [18]. Additionally, malignancy rates of 34.9% and 39% have also been reported [8,19]. In the current study, the malignancy rate among Bethesda III nodules was 35.3%, comparable to what has been reported in the literature.
In a study by Bresler et al., 9% of Bethesda III nodules were histologically malignant, with 50% of these being PTC and 30% PTMC [20]. Another study found PTC as the predominant histological type among malignant TNs [8], while Finlayson et al. reported follicular carcinoma as the predominant type [21]. In this study, PTC and PTMC were the most common cancer types among the malignant nodules, with equal incidence (15.9%).
The primary objective of thyroid surgery for nodules classified as AUS/FLUS is to achieve a definitive histological diagnosis while ensuring complete removal of the pathological nodule. This approach aims to facilitate optimal surgical and medical management, thereby minimizing the risks associated with excessive surgical intervention and related adverse events [1]. Surgical options for TNs, such as total thyroidectomy, lobectomy, or nodulectomy, are influenced by several factors. These include risk factors indicating a higher likelihood of malignancy (such as nodules larger than 4 cm, a family history of neoplasia, and a history of radiation exposure), U/S characteristics, cytological category, and molecular testing. Additionally, these risk factors should be considered in conjunction with the patient’s preferences, the presence of contralateral nodularity, possible coexisting hyperthyroidism, and any comorbid conditions [22]. It is important to note that total thyroidectomy is no longer universally recommended for all differentiated carcinomas larger than 1 cm. According to the 2015 ATA Guidelines, lobectomy may be an adequate initial treatment for differentiated carcinomas smaller than 4 cm, NIFTP of any size, minimally invasive follicular carcinomas, and encapsulated or intrathyroidal variants of papillary carcinomas [1,22]. Another study recommended that nodulectomy may be an appropriate option for managing large, solitary TNs and small suspicious nodules or microcarcinomas [23]. In the present study, 67.6% of the cases underwent total thyroidectomy, with lobectomy in 17.6%, nodulectomy in 12.4%, and isthmusectomy in 2.4%. The present study's limitations included a small sample size and the retrospective nature of data collection, which may have led to the omission of important data, such as details on ultrasonography.
Conclusion
One of three TNs with indeterminate cytology may be malignant. Patients with malignant nodules tend to be younger than those with benign nodules, and benign nodules could be larger. While total thyroidectomy is common, lobectomy and nodulectomy may be viable alternatives for specific cases, emphasizing the need for individualized treatment.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The study's ethical approval was obtained from the scientific committee of the Kscien Organization for Scientific Research.
Patient consent (participation and publication): Verbal informed consent was obtained from patients for publication.
Source of Funding: Smart Health Tower.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: AMS and AMA were significant contributors to the conception of the study and the literature search for related studies. RMA, AJQ, ROM and RJR were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. HOA, HOB, and AAQ were involved in the literature review, study design, and manuscript writing. HAA and SHH Literature review, final approval of the manuscript, and processing of the tables. RMA was the pathologist who performed the histopathological diagnosis. HOA and RMA confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.
References
- nodules with indeterminate cytology: Association between nodule size, histopathological characteristics and clinical outcome in differentiated thyroid carcinomas—A multicenter retrospective cohort study on 761 patients. Updates in Surgery. 2021;73(5):1923-30. doi:10.1007/s13304-021-01096-2
- Naidu K, Saksenberg V, Mahyoodeen NG. Clinical and ultrasound characteristics distinguishing benign and malignant thyroid nodules in Johannesburg, South Africa. Journal of Endocrinology, Metabolism and Diabetes in South Africa. 2023;28(2):62-8. doi:10.1080/16089677.2023.2178274
- Dimitriadis PA, Moinie A, Michaels J, Bance R, Vijendren A, Mochloulis G. Indeterminate thyroid nodules (Thy3): malignancy rate and characteristics in a study of 118 patients. The Annals of The Royal College of Surgeons of England. 2023;105(6):568-71. doi:10.1308/rcsann.2022.0163
- Chen D, Hu J, Zhu M, Tang N, Yang Y, Feng Y. Diagnosis of thyroid nodules for ultrasonographic characteristics indicative of malignancy using random forest. BioData mining. 2020; 13:1-21. doi:10.1186/s13040-020-00223-w
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341-6. doi:10.1089/thy.2017.0500
- Kakamad FH, Abdalla BA, Abdullah HO, Omar SS, Mohammed SH, Ahmed SM, et al. Lists of predatory journals and publishers: a review for future refinement. European Science Editing. 2024;50: e118119. doi:10.3897/ese.2024.e118119
- Luo W, Yang X, Yuan J, Pang L, Zhang P, Ding L, et al. Evaluation of characteristics of thyroid nodules on contrast-enhanced ultrasonography: a retrospective analysis of 252 cases. Medical Ultrasonography. 2020;22(2):164-70. doi:10.11152/mu-2252
- Rano E, Lin L, Molinie V, Sulpicy C, Dorival MJ, Drak Alsibai K, et al.Epidemiological, Clinical, Ultrasonographic and Cytological Characteristics of Thyroid Nodules in an Afro-Caribbean Population: A Series of 420 Patients. Cancers. 2022;14(10): 2365. doi:10.3390/cancers14102365
- Bessey LJ, Lai NB, Coorough NE, Chen H, Sippel RS. The incidence of thyroid cancer by fine needle aspiration varies by age and gender. Journal of Surgical Research. 2013;184(2):761-5. doi:10.1016/j.jss.2013.03.086.
- Rago T, Scutari M, Latrofa F, Loiacono V, Piaggi P, Marchetti I, et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome, and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. The Journal of Clinical Endocrinology & Metabolism. 2014;99(10):3700-7. doi:10.1210/jc.2013-4401.
- Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom national multidisciplinary guidelines. The Journal of Laryngology & Otology. 2016;130(S2):S150-60. doi:10.1017/S0022215116000578.
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009 ;19(11):1167-214. doi:10.1089/thy.2009.0110.
- Kiernan CM, Solórzano CC. Bethesda category III, IV, and V thyroid nodules: can nodule size help predict malignancy? Journal of the American College of Surgeons. 2017;225(1):77-82. doi:10.1016/j.jamcollsurg.2017.02.002.
- Talmor G, Badash I, Zhou S, Kim YJ, Kokot NC, Hsueh W, et al. Association of patient characteristics, ultrasound features, and molecular testing with malignancy risk in Bethesda III–V thyroid nodules. Laryngoscope Investigative Otolaryngology. 2022;7(4):1243-50. doi:10.1002/lio2.847.
- Mihai R, Parker AJ, Roskell D, Sadler GP. One in four patients with follicular thyroid cytology (THY3) has a thyroid carcinoma. Thyroid. 2009;19(1): 33-7. doi:10.1089/thy.2008.0200
- Cavallo A, Johnson DN, White MG, Siddiqui S, Antic T, Mathew M, et al. Thyroid nodule size at ultrasound as a predictor of malignancy and final pathologic size. Thyroid. 2017;27(5):641-50. doi:10.1089/thy.2016.0336.
- Chadwick D, Kinsman R, Walton P. Fifth National audit report. British Association of Endocrine and Thyroid Surgeons, Henley-on-Thames: Dendrite Clinical Systems. 2017. https://www.baets.org.uk/wp-content/uploads/BAETS-Audit-National-Report-2017.pdf.
- Poller DN, Bongiovanni M, Trimboli P. Risk of malignancy in the various categories of the UK Royal College of Pathologists Thy terminology for thyroid FNA cytology: A systematic review and meta‐analysis. Cancer cytopathology. 2020;128(1):36-42. doi:10.1002/cncy.22201
- Calò PG, Medas F, Santa Cruz R, Podda F, Erdas E, Pisano G, et al. Follicular nodules (Thy3) of the thyroid: is total thyroidectomy the best option? BMC surgery. 2014; 14:1-7. doi:10.1186/1471-2482-14-12
- Bresler A, Mehta V, Schiff BA, Smith RV, Khader S, Ramos‐Rivera G, et al. Comparison of Bethesda cytopathology classification to surgical pathology across racial‐ethnic groups. Head & Neck. 2019;41(7):2340-5. doi:10.1002/hed.25707.
- Finlayson A, Barnes I, Sayeed S, McIver B, Beral V, Ali R. Incidence of thyroid cancer in England by ethnic group, 2001–2007. British journal of cancer. 2014;110(5):1322-7. doi:10.1038/bjc.2014.4.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-33. doi:10.1089/thy.2015.0020.
- Salih AM, Muhialdeen AS, Ismaeil DA, Saeed YA, Dhahir HM, Baba HO, et al. Thyroid nodulectomy: A promising approach to the management of solitary thyroid nodules. Biomedical Reports. 2024;21(2):1-6. doi:10.3892/br.2024.1805.

This work is licensed under a Creative Commons Attribution 4.0 International License.

