Blood Cross Matching Without Anti-Human Globulin (AHG) and Bovine Serum: A New Interest for an Old Idea
Abstract
Introduction
Transfusion medicine promotes the safety of blood transfusions by rigorously testing to eliminate risks of infection and hemolytic. The efficacy (to correct and identify antibodies) of a modified cross-matching method that excludes the use of anti-human globulin (AHG) bovine serum with that of the immediate spin crossmatch was assessed.
Material and Methods
This multi-center study was performed at two medical centers in Iran and Iraq. Over seven years, consecutive participants received two different blood cross-matching methods: one with AHG and bovine serum and another without it.
Results
The study included 31240 participants. About 18526 (59.3%) were males and 12714 (40.7%) were females. The ages of participants ranged from 23 to 57 years, with the average age of 39 years. Only 45 (0.14%) participants blood cross-match incompatible in both spin cross-match and cross-match without AHG, bovine serum the same result. The study found that all detected antibodies correlated with potential blood incompatibility, and there remained 100% safety between the two testing methods.
Conclusion
Omitting AHG and bovine serum in cross-matching might be safe. At least it can be used in emergency situations and in resource-limited settings.
Introduction
A transfusion medic is in charge of making sure the blood you are being transfused is being transfused as safely as possible. To address concerns such as transfusion-transmitted infections (TTIs) and transfusion-associated hemolytics [1], an exhaustive testing procedure is performed between the donor and recipient blood. The present red cell serology based on safe transfusion was developed in 1901 with Karl Landsteiner's identification of the ABO blood types. Later, in that same year, 1940, the Rh antigen and in the year 1946, the Kell antigen were shown to be significant red cell antigens [2]. Provided donor and recipient blood types are compatible, these minimally increase the risks of medical procedure complications, adverse reactions, and other conditions that benefit from medical procedures such as organ transplants [3,4]. Cross-matching aims to see if the blood types from the donor and receiver are compatible [5]. This is done to avoid potentially fatal immunological responses to transfused blood. This approach can search the recipient's blood for antibodies that are incompatible with the red blood cell antigens in the donor’s cells [6,7]. IgM or IgG are usually blood group antibodies, but occasionally IgA. In most cases, immune antibodies (generally IgG) and naturally occurring antibodies (predominantly IgM) [8]. The Anti human Globulin (AHG) and bovine serum detect weak antibodies, such as most IgG and some IgM antibodies. However, the bovine serum also detects weak antibodies such as IgG due to increasing its dielectric constant and hence decreasing the Zeta potential, which causes the red cell to come closer, thereby decreasing the thickness of the ionic cloud surrounding each cell, resulting in agglutination [9]. AHG helps reveal the presence of antibodies that may have coated the surface of red blood cells even when agglutination is not apparent during the initial testing phases; adding AHG and bovine to the test significantly increases the test's sensitivity [10]. These antibodies may not cause visible clumping or agglutination on their own; however, not all antigens lead to the formation of clinically significant antibodies to identify the 25–28 blood group antigens that are known to elicit hemolytic reactions (HTRs), screening for significant antibodies in the blood cells is recommended [11]. Antibody screening tests are of significant use in both the diagnosis of infectious diseases and the testing of immune systems, as well as the discovery of autoimmune conditions that can be gained from the use of antibody screening tests. As part of this procedure, the majority of blood banks routinely perform a battery of tests to identify the ABO and Rh types of both the donor and the recipient and then carry out a full cross-match of the recipient’s serum with the donor’s red blood cells to ensure transfusion stability, at times being an integral part of this procedure is a blood cross-match with bovine serum plus AHG.
The objective of the study was to assess the possibility of making the blood cross machining method safe and accurate, when AHG and bovine serum were not utilized in the process.
Material and Methods
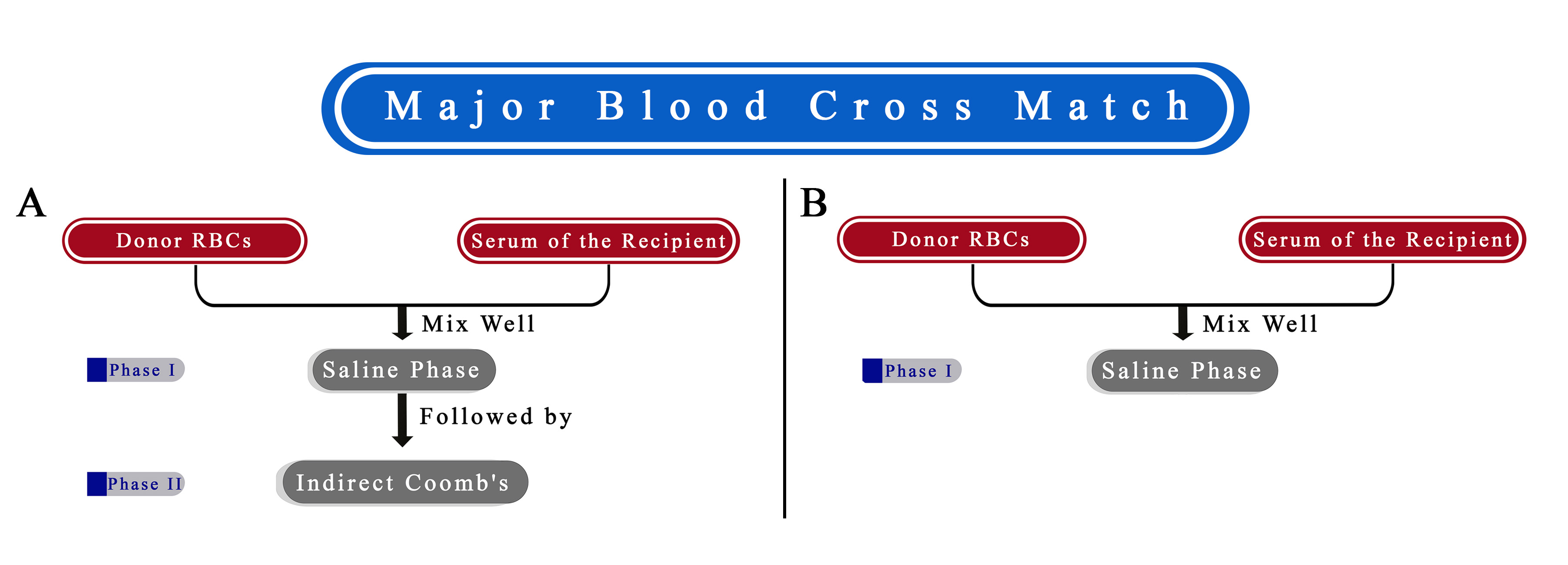
This multi-center cohort study was conducted at the Blood Bank Department of General Hospital in Kurdistan, Iraq, and Kawsar Hospital in Sanandaj, Iran. The research spanned seven years (from December 2017 to November 2024). All patients necessitated blood transfusions, and some volunteers participated in the spin cross-match (blood cross-match with AHG, bovine) [13]. All tests were repeated blood cross-matches without AHG, bovine (Figure 1). (Inclusion criteria: patients and volunteers must possess identical ABO and Rh blood types.
Only 45 participants (1.4%) exhibited blood cross-match incompatibility in both spin cross-match and cross-match without AHG and bovine; the same result was observed where they tested positive for antibodies. Antibody screening for incompatible patients was conducted using the Bio-Rad ZE5 Cell Analyzer, a high-performance flow cytometry platform designed for a diverse array of cellular assays, providing advanced capabilities for researchers in immunology and oncology. It combines flexibility with high throughput. Data analysis each variable underwent a descriptive analysis, calculating percentages, frequencies, means, and ranges. The data were gathered on an Excel sheet. Whenever necessary, the P-value significance was set to less than 0.05.
Results
The study included 31240 participants. About 18526 (59.3%) were males and 12714 (40.7%) were females. Participants’ ages ranged from 23 to 57 years, with an average age of 39. Only 45 (1.4%) participants' blood cross was incompatible in spin cross-match and cross-match without AHG; bovine serum. From this number, 27 (53.3%) had a history of blood transfusion, 16 (59% male), and 11 (41% female). About 18 (46.7%) of women who have been pregnant at least once are included [table 1].
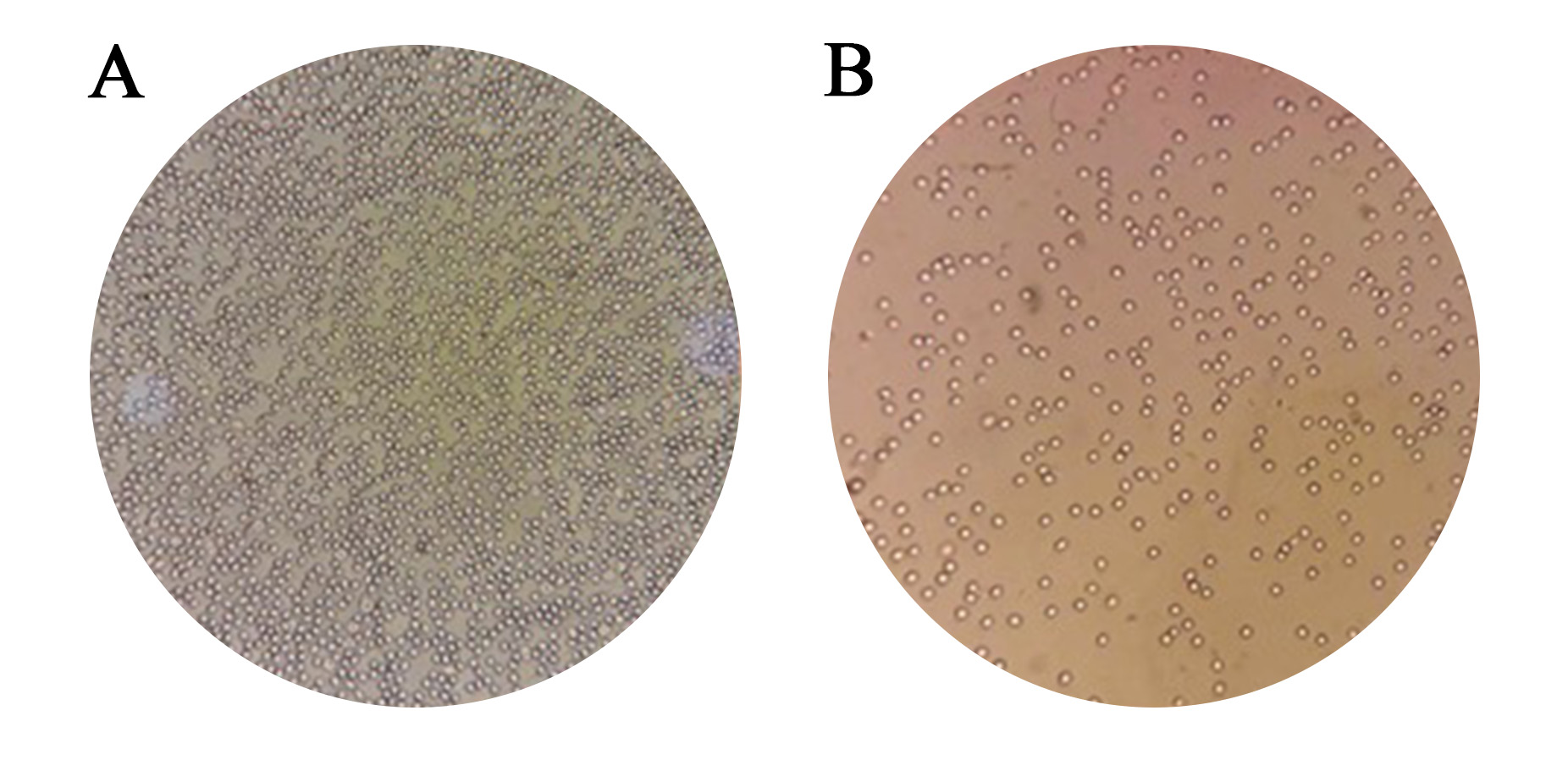
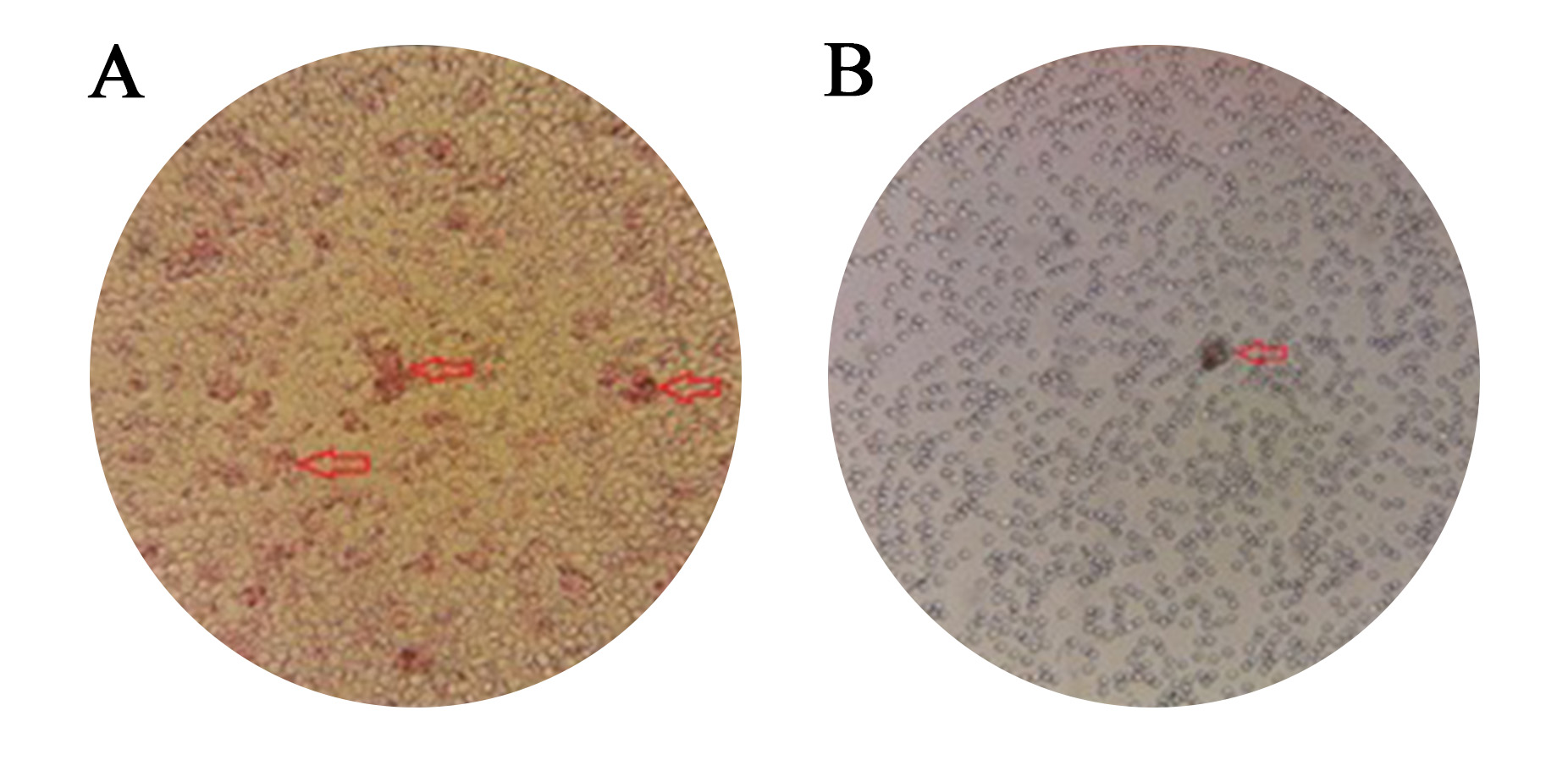
The results of the two tests about compatibility were identical in every way [Figure 2 and 3]. No discrepancy was found between the two procedures.
|
Alloantibodies |
Frequency (%) |
|
Anti-D |
16 (35.5%) |
|
Anti-M |
11 (24.4%) |
|
Anti-C |
9 (20%) |
|
Anti-E |
2 (4.4%) |
|
Anti-Jkb |
2 (4.4%) |
|
Anti-S |
1 (2.2%) |
|
Anti-Lea |
1 (2.2%) |
|
Anti-K |
1 (2.2%) |
|
Anti-Cw |
1 (2.2%) |
|
Anti-N |
1 (2.2%) |
|
Total |
45 (100%) |
Discussion
The International Society of Blood Transfusion has recognized 33 blood group systems, and that's a significant step forward. Other than the ABO and Rhesus systems, red blood cell membranes contain a plethora of different antigens. This is important to avoid transfusion-related problems [14]. One of the common hazards associated with red blood cell transfusions is the formation of RBC alloantibodies. Red blood cell alloimmunisation rates are notable in 1% to 35% of some groups of patients [15,16]. In this study, the alloimmunisation rate was far lower at 0.14%. The distribution of alloantibodies was determined in 35.5% (16 out of 45) of the cases where anti-D was found. About 24.4% (11 out of 45) were anti-M. The third most common group was Anti-C antibodies, at about 20%.
In Western nations, studies have shown a much higher incidence of Kell antibodies [16], which contradicts our results. This might need further studies with a broader population. The striking finding of this study was that there was not a single case where the spin cross was compatible, but without AHG, the bovine cross-match was incompatible, which is an important finding. The screening cells found every clinically relevant antibody. After the cross-match method, it was found that this screening panel is suitable for use in Iranian and Iraqi blood banks, at least in special situations like emergency settings or resource-limited regions.
This demonstrates that if the AHG bovine had been deleted from the blood and the blood was supplied using this approach, an identical result would have been observed using the AHG bovine in vitro. Identifying all clinically important antibodies is a crucial part of the pre-transfusion testing process and helps avoid an adverse response. The findings of the test are interpreted according to the presence or lack of agglutination or other markers of antigen binding [17].
On the other hand, eliminating the traditional AHG, bovine serum cross-match, may be advantageous. There is a reduction in the amount of labor that has to be done, a reduction in the prices of the reagents, a straightforward and speedy method, suitableness for emergencies, sensitivity and accuracy, fast detection of incompatible conditions, and more efficient utilization of blood inventory.
The technical staff will not have to carry out an AHG, bovine serum cross-match every ten to fifteen minutes. As a result, they will have more time to dedicate to other regions, such as donor recruiting and well-being. This cross-match without AHG bovine serum is also excellent for the development of a blood bank. A procedure for computer cross-match might potentially be created with the confidence obtained from this work in the future, provided that the software and checking points that are used are confirmed. However, confirming these benefits needs several better-designed studies with more variables.
This study has several limitations: First, although the sample size is reasonable, there is no significant data regarding the cost of the procedures in both situations. Second. The duration of both tests has not been reported. Third, we failed to report the number of agglutinations found on each test plate.
Conclusion
Based on this study's findings, crossmatching without the use of AHG and bovine serum demonstrated a predicted safety rate of 100%, yielding results comparable to those of the spin crossmatch method. This suggests that omitting AHG and bovine serum in crossmatching may be a safe alternative, particularly in emergencies and resource-limited settings, where simplifying procedures can help optimize care without compromising safety.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: The ethics board of the Kurdistan University of Medical Science approved the study.
Patient consent (participation and publication): Written informed consent was obtained from patients for publication.
Source of Funding: Kurdistan University of Medical Science.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: MRR significantly contributed to the study's conception and literature search for related studies. SHK and MB were involved in the literature review, the study's design, and the critical revision of the manuscript. They also participated in data collection, the literature review, study design, and manuscript writing. MRR and SHK confirmed the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.
References
- K. Landsteiner and A. S. Wiener, "An Agglutinable Factor in Human Blood Recognized by Immune Sera for Rhesus Blood," Experimental Biology Medicine, vol. 43, no. 1, doi:10.3181/00379727-43-11151, 1940.
- D. W. Greening, K. M. Glenister, R. L. Sparrow, and R. J. Simpson, "International blood collection and storage: Clinical use of blood products," Journal of Proteomics, vol. 73, no. 3, pp. 386-395, doi:10.1016/j.jprot.2009.07.011, 2010.
- L. Lögdberg, M. E. Reid, and T. Zelinski, "Human blood group genes 2010: Chromosomal locations and cloning strategies revisited," Transfus Med Rev, vol. 25, no. 1, pp. 36-46, doi:10.1016/j.tmrv.2010.08.005, 2011.
- S. F. Ghirardo, I. Mohan, A. Gomensoro, and M. I. Chorost, "Routine preoperative typing and screening: A safeguard or a misuse of resources," JSLS, vol. 14, no. 3, pp. 395-398, doi:10.4293/108680810X12924466007241, 2010.
- L. Onotai and O. D. Lilly-Tariah, "Adenoid and tonsil surgeries in children: How relevant is pre-operative blood grouping and cross-matching?" Afr J Paediatr Surg, vol. 10, no. 3, pp. 231-234, doi:10.4103/0189-6725.120887, 2013.
- R. Mitra, N. Mishra, and G. P. Rath, "Indian J Anaesth," vol. 58, no. 5, pp. 524–528, DOI: 10.4103/0019-5049.144645, 2014.
- J. Park and J.-K. Park, "Finger-actuated microfluidic device for the blood cross-matching test," Lab on a Chip, vol. 18, no. 8, pp. 1215-1222, doi:10.1039/C7LC01128H, 2018.
- J. W. Loughney, C. Lancaster, S. Ha, and R. R. Rustandi, "Residual bovine serum albumin (BSA) quantitation in vaccines using automated Capillary Western technology," Analytical Biochemistry, vol. 46, no. 3, pp. 49-56, doi:10.1016/j.ab.2014.05.004, 2014.
- K. Wu, S. Duan, Y. Wang, H. Wang, and X. Gao, "Convolutional neural network-based automatic classification for incomplete antibody reaction intensity in solid phase anti-human globulin test image," International Federation for Medical and Biological Engineering, vol. 60, no. 7, pp. 1211–1222, doi:10.1007/s11517-022-02523-1, 2022.
- J. Adez, J. Cronin, R. S. Green, A. M. Fernánberg, and E. S. Heitmiller, "Pediatric preoperative blood ordering: when is a type and screen or crossmatch really needed?" Pediatric Anesthesia, vol. 33, no. 9, pp. 146-150, doi:10.1111/pan.12250, 2023.
- Erwin Strobel; Hemolytic Transfusion Reactions. Transfus Med Hemother. 2008; 35 (5): 346–353. doi:10.1159/000154811.
- Schonewille H., Brand A. Allo-immunization to red blood cell antigens after universal leuco-depletion: A regional multi centre retrospective study. Br J Haematol 2005; 129:51–6. doi:10.1111/j.1365-2141.2005.05408.x
- Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Allo-immunization and erythrocyte auto-immunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood 2000; 96:3369-73. doi:10.1182/blood.V96.10.3369
- Makarovska-Bojadzieva T, Blagoevska M, Kolevski P, Kostovska S. Optimal blood grouping and antibody screening for safetransfusion. Prilozi 2009; 30:119-28. doi:N/A
- R. Mitra, N. Mishra, and G. P. Rath, "Blood groups systems," Indian J Anaesth, vol. 58, no. 5, pp. 524-528, doi:10.4103/0019-5049.144645, 2014.
- T. C. Hall, C. Pattenden, C. Hollobone, C. Pollard, and A. R. Dennison, "Blood Transfusion Policies in Elective General Surgery: How to Optimise Cross-Match-to-Transfusion Ratios," Transfus Med Hemother, vol. 40, no. 1, pp. 27–31, doi:10.1159/000345660, 2013.
- T. Bartolmäs, B. Mayer, S. Yürek, R. Genth, and A. Salama, "Paradoxical findings in direct antiglobulin test and classification of agglutinating autoantibodies using eluates and monospecific anti-human globulin sera," The International Journal of Transfusion Medicine, vol. 108, no. 1, pp. 58-63, doi:10.1111/vox.12187, 2014.

This work is licensed under a Creative Commons Attribution 4.0 International License.

